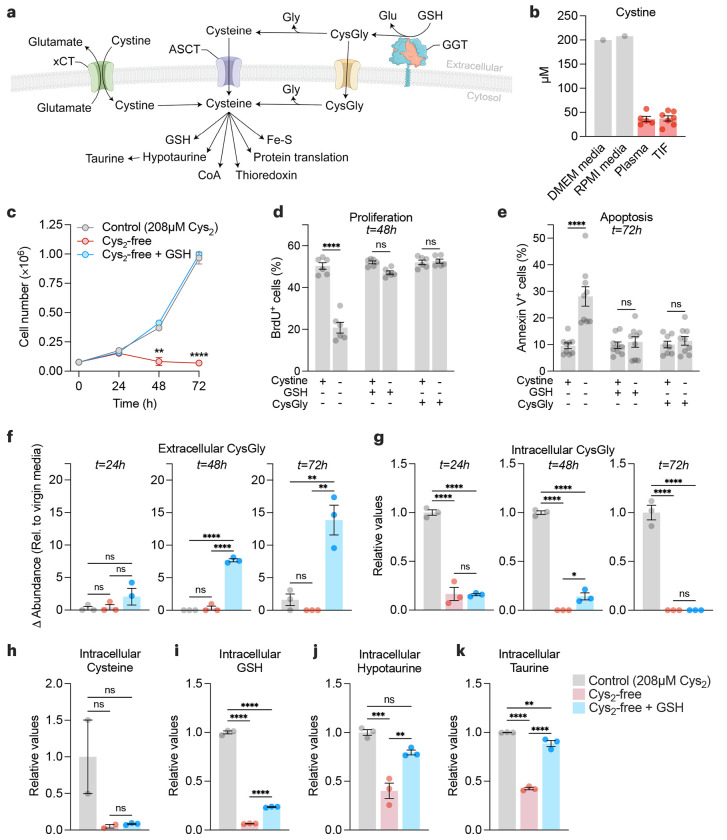
Figure 2. Extracellular GSH supplies amino acids to promote cancer cell growth and survival in cystine-free environments.
a, Schematic of the different mechanisms of cysteine acquisition and utilization. b, Concentration of cystine in cell culture media formulations (grey bars), and in tumor interstitial fluid (n=7) and matched plasma (n=5) from Balb/c mice bearing 4T1 orthotopic allografts (red bars). c-e, HCC-1806 breast cancer cells were grown in control (208 μM cystine; Cys2), cystine-free (Cys2-free), cystine-free/GSH-supplemented (750 μM) or cystine-free/CysGly-supplemented (750 μM) medium and cell numbers (c), percentages of proliferative (BrdU+) (d) and apoptotic (Annexin V+) (e) cells was determined at indicated timepoints. Statistical significance was analyzed by two-way ANOVA followed by Tukey’s multiple comparisons test (n=4 independent experiments) for (c), two-way ANOVA followed by Šídák’s multiple comparisons test (n=3 independent experiments) for (d-e). f-g, Levels of extracellular CysGly in media (f) and intracellular CysGly in HCC-1806 breast cancer cells (g) grown in indicated media and at indicated time points. h-k, Levels of intracellular cysteine (h), GSH (i), hypotaurine (j), and taurine (k) in indicated media and at indicated time points. Statistical significance was assessed by one-way ANOVA followed by Tukey’s multiple comparisons test. Data is representative of an experiment with 3 biological replicates and is represented as mean ± s.e.m., *p-value<0.05; **p-value<0.01; ***p-value<0.001; ****p-value<0.0001; ns, not significant.