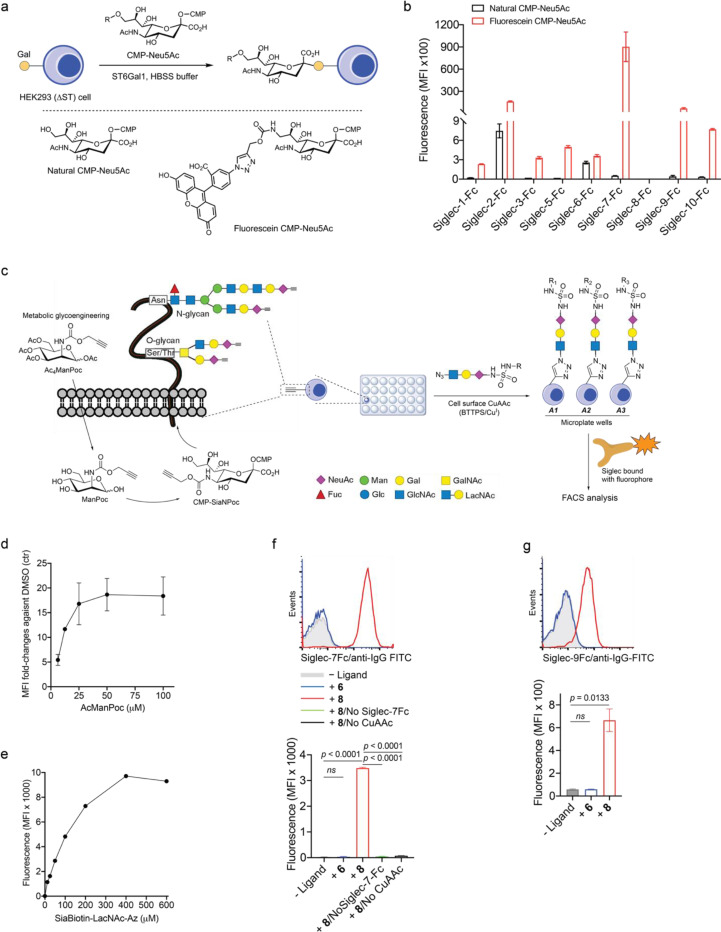
Extended Data Fig. 5 |. Design and synthesis of high-affinity and selective Siglec ligands via cell-surface CuAAC assay.
a,b, Evaluation of cross-binding of a reported Siglec-7 high-affinity ligand (fluorescein-modified Neu5Ac) towards a panel of human Siglecs. Fluorescein-Neu5Ac was transferred from the CMP-fluorescein-Neu5Ac donor to cell surface of sialic acid-depleted HEK293 (ΔST) cells using recombinant ST6Gal1 in HBSS buffer (a). Neu5Ac-installed cells were probed with fluorophore-bound Siglec-Fc chimeras for flow cytometry analysis of binding events (b). ST, sialyltransferase. c, General scheme for biocompatible cell-surface Neu5Ac ligand screening assay enabled by BTTPS-accelerated CuAAC, in which, Jurkat cells (No Siglec expression) that were metabolically labeled with Ac4ManPoc in complete growth media to incorporate alkynylated sialic acid onto the cell surface, were seeded to 96-well microplate in PBS buffer containing 1% FBS, pre-mixed CuSO4/BTTPS and Neu5Ac-LacNAc-azide ligands, followed by sodium ascorbate to initiate CuAAC to install Neu5Ac ligands onto the cell surface in a multivalent context. The modified cells were probed with fluorophore-bound Siglec-Fc chimeras for flow cytometry analysis. Man, mannose; GalNAc, N-acetyl-galactosamine; Fuc, fucose; Glc, glucose; Ac4ManPoc, N-propargyloxycarbamate-1,3,4,6-tetra-O-acetyl-manosamine; CuSO4, copper(II) sulfate; CuAAc, Cu(I)-catalyzed azide–alkyne cycloaddition; BTTPS, 3-[4{(bis[(1-tert-butyl-1H-1,2,3-triazol-4-yl)methyl]amino)methyl}1H-1,2,3-triazol-1-yl]propyl hydrogen sulfate; FACS, fluorescence-activated cell sorting. d, Jurkat cells were incubated with Ac4ManPoc at various doses for 3 days, followed by conjugation with biotin azide via cell-surface BTTPS-accelerated CuAAc and staining with APC streptavidin for flow cytometry analysis. Biotin azide, PEG4 carboxamide-6-azidohexanyl biotin. e, Alkyne-labeled Jurkat cells were reacted with biotinylated Neu5Ac-LacNAc-azide at different concentrations via BTTPS-accelerated CuAAc, followed by staining with APC streptavidin for flow cytometry analysis. f,g, Histogram and MFI analysis of benzothiazole-modified Neu5Ac-LacNAc ligand (8) installed on Jurkat cells probed with the recombinant Siglec-7 and -9 Fc chimeras (based on the optimized conditions from c,d,e) in comparison with ‘No ligand’ treatment and natural Neu5Ac-LacNAc (6), in the presence or absence CuAAc click chemistry. The elimination of individual components in the binding assay completely negated the observed binding events, validating their authenticity (f). Data are mean ± s.d. Two-tailed unpaired Student’s t-test. ns, not significant (f,g).