Abstract
OBJECTIVES
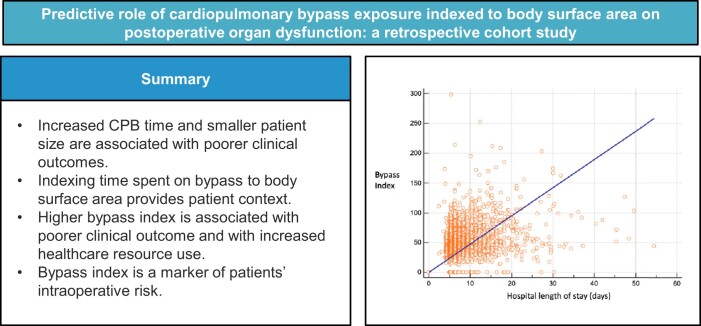
Long cardiopulmonary bypass times are associated with adverse postoperative outcomes and increased healthcare resource use. It is likely that this effect is pronounced in smaller patients. Previous studies have been criticized for not taking into consideration that prolonged bypass times are often due to higher complexity. The purpose of this study was to investigate the relationship between bypass index (bypass time/body surface area) and adverse postoperative events.
METHODS
Single-centre, retrospective cohort study including 2413 patients undergoing cardiac surgery on cardiopulmonary bypass from June 2018 to April 2020. Length of hospital stay, as surrogate marker of postoperative morbidity, was selected as primary outcome. The strength of association between bypass index and the primary outcome was assessed with linear regression analysis. Secondary outcomes included new onset renal, pulmonary or cardiac rhythm dysfunction. The predictive value of bypass index was assessed with linear regression analysis; univariate and multiple regression were used to assess the strength of association between Bi and the secondary outcomes.
RESULTS
Bypass index was predictive for length of stay at univariate (Relative Risk (RR): 1.004, P < 0.001) and at multivariable (RR: 1.003, P < 0.001) analysis. The association between bypass index and new renal (mean difference: 14.1 min/m2, P < 0.001) and cardiac rhythm dysfunction (mean difference: 12.6 min/m2) was significant. This was not true of postoperative lung dysfunction (mean difference: −1.5 min/m2, P = 0.293).
CONCLUSIONS
Bypass index, calculated as total bypass time/patient body surface area, is predictive of postoperative morbidity and resource utilization after cardiac surgery on pump.
Keywords: Cardiopulmonary bypass, Organ dysfunction, Acute kidney injury, Atrial fibrillation, Length of stay
Since its inception in the 1950s, cardiopulmonary bypass (CPB) has been an essential component of cardiac surgery.
Graphical Abstract
INTRODUCTION
Since its inception in the 1950s, cardiopulmonary bypass (CPB) has been an essential component of cardiac surgery. Early experience demonstrated an association between CPB exposure and postoperative organ dysfunction, morbidity and mortality [1–3]. CPB has been shown to activate coagulation, fibrinolysis [4] and platelets [5]. Multiple inflammatory mediators have been implicated in contributing to organ dysfunction [6]; key drivers for the inflammatory response appear to be air-blood interface [7], circuit artificial surface [8] and re-infusion of shed pericardial blood [9].
Longer CPB time has been associated with increased duration of mechanical ventilation [10] and higher risk of surgical site infection [11], kidney injury [12], organ failure [13], and mortality [14]. It is conceivable that the risk of CPB-associated complications correlates inversely with patient size. Lower body surface area (BSA) is associated with increased haemodilution at CPB initiation [15] and increased odds of allogeneic red blood cell transfusion [16]. High body mass index (BMI) has been associated with lower odds of mortality in patients undergoing cardiac surgery [17].
The purpose of the current study is to investigate the role of a bypass index (BI), calculated as total CPB time divided by the patient BSA. To the best of our knowledge, no prior study has investigated the predictive role of bypass time indexed to patient size on postoperative morbidity and mortality, despite biological plausibility.
METHODS
This study was designed utilizing the STROBE checklist for observational studies [18]. We conducted a single-centre, retrospective, cohort study of adult patients (18 years and over) undergoing elective or semi-urgent cardiac surgery on CPB at an academic, quaternary referral hospital between 1 June 2018 and 30 April 2020. Patients having deep hypothermic circulatory arrest, solid organ transplant, major aortic surgery, pulmonary thrombo-endarterectomy, urgent (<8 h) and emergent surgery (<4 h) were excluded as were those in end-stage renal failure requiring haemodialysis and those who died within the first 30 days after surgery. Anaesthesia and intensive care data were retrieved from Metavision (iMDsoft, Duesseldorf, Germany), and patient demographics data, risk and surgical data were retrieved from an in-house database, CARDS II.
The key exposure of interest was the BI. The primary outcome was the strength of association between BI and hospital length of stay (LOS) as a surrogate marker for morbidity (Table 1). Secondary outcomes included the strength of association between BI and new renal (creatinine rise >50% on postoperative day 1), pulmonary (PaO2/FiO2 <200 during the first 6 h postoperatively) and cardiac rhythm dysfunction (new-onset atrial fibrillation within 24 h postoperatively), intensive care unit (ICU) LOS. We included a pre-specified subgroup of patients undergoing isolated coronary artery bypass grafting (CABG) and undertook a post hoc analysis assessing the primary outcome with CPB time only as the exposure of interest to investigate whether BI provides additional value as compared to CPB time alone.
Table 1:
Hospital length of stay (death within 30 days excluded)
| Univariate beta coefficient (95% CI) | P-value | Multivariable beta coefficient (95% CI) | P-value | |
|---|---|---|---|---|
| BI (per unit) | 0.004 (0.003, 0.005) | <0.001 | 0.034 (0.027, 0.094) | <0.001 |
CI: confidence interval; BI: bypass index.
Anaesthesia and perfusion technique
Please see the Supplementary material.
Statistical methods
Continuous variables are presented as mean ± standard deviation (SD) and median—interquartile range (IQR) for normally and non-normal distributed data, respectively. Categorical variables are reported as counts and percentages.
The strength of association between BI or CPB time and LOS was assessed using negative binomial regression analysis. Unadjusted comparison of means, univariate and multiple regression analyses were carried out to examine whether BI is associated with new postoperative cardiac rhythm disturbance, new renal dysfunction, new pulmonary dysfunction and 30-day mortality. Variables included in multivariable regression analyses were BI, sex, age and BMI. Where regression analyses were undertaken, binary outcome variables were reported using the odds ratio, and continuous outcome variables using the beta coefficient.
Following first analysis we investigated the association between ICU and hospital LOS and BI. Generalized additive model (GAM) framework was used to model potential non-linear relationships and interactions between predictors. GAMs were fitted using the geom_smooth() function in ggplot2 with method = ‘gam’, incorporating a smooth term for BI to capture non-linear effects of the continuous predictor BI, and categorical predictor of comorbidities representing clinical presentations of pulmonary, cardiac and renal dysfunction as well as combinations of them. Model parameters were automatically adjusted through penalized likelihood estimation, ensuring robustness in modelling complex relationships.
Secondary analyses were undertaken to further investigate any association between BI and postoperative organ dysfunction in patients undergoing CABG only.
Statistical analysis was performed using the Stata software package (StataCorp, College Station, TX, USA) and in the statistical programming environment R Version 4.4.1 (Vienna, Austria) using the mgcv package where necessary and ggplot2 for visualization of results.
RESULTS
From 1 June 2018 to 30 April 2020, 2413 patients met the inclusion criteria. 119 patients were excluded due to missing data (14 due to missing BSA data, 105 due to missing outcome data). 2294 patients were included in our final analysis. Demographic is summarized in Table 2.
Table 2:
Summary statistics
| Male, n (%) | 1687 (26.6%) |
| Female, n (%) | 610 (26.5%) |
| Age, mean (SD) | 68.8 (11.1) |
| Height (cm), mean (SD) | 169.9 (9.5) |
| Weight (kg), mean (SD) | 82.6 (16.8) |
| BSA, mean (SD) | 1.97 (0.23) |
| EuroSCORE 2, mean (SD) | 3.15 (4.35) |
| Preoperative rhythm | |
| Sinus, n (%) | 1897/2271 (82.6%) |
| AF/flutter, n (%) | 314/2271 (13.8%) |
| Other, n (%) | 60/2271 (2.6%) |
| Type of surgery | |
| CABG, n (%) | 840/2297 (36.6%) |
| Valve, n (%) | 690/2297 (30.0%) |
| Double valve, n (%) | 105/2297 (4.6%) |
| Triple valve, n (%) | 11/2297 (0.5%) |
| CABG + valve, n (%) | 404/2297 (17.6%) |
| Aortic, n (%) | 167/2297 (7.3%) |
| Redo, n (%) | 59/2297 (2.6%) |
| Other, n (%) | 21/2297 (0.9%) |
| Total bypass time (min), mean (SD) | 108.7 (57.5) |
| Bypass index, mean (SD) | 55.9 (31.2) |
| Any organ dysfunction, n (%) | 960/2297 (41.8%) |
| New postoperative rhythm dysfunction, n (%) | 304/2288 (13.3%) |
| Postoperative renal dysfunction, n (%) | 90/2276 (4%) |
| Postoperative CRRT, n (%) | 31/2297 (1.3%) |
| Postoperative pulmonary dysfunction, n (%) | 724/2293 (31.5%) |
SD: standard deviation; BSA: body surface area; AF: atrial fibrillation; CABG: coronary artery bypass surgery; CRRT: continuous renal replacement therapy.
Primary outcome
Primary outcome analyses demonstrated predictive utility of BI on hospital LOS at both univariate (beta coefficient: 0.004 days per BI unit, 95% CI: 0.003–0.005, P < 0.001) and multivariable (beta coefficient: 0.003 days per BI unit, 95% CI: 0.002–0.004, P < 0.001) levels.
Supplementary Material, Table S1 presents a comparison of the baseline and the postoperative variables between patients with hospital LOS < median and those with hospital LOS ≥ median. This comparison confirms that patient factors such as age as well as intraoperative factors such as CPB time and BI or postoperative factors such as organ dysfunction are all associated with increased LOS.
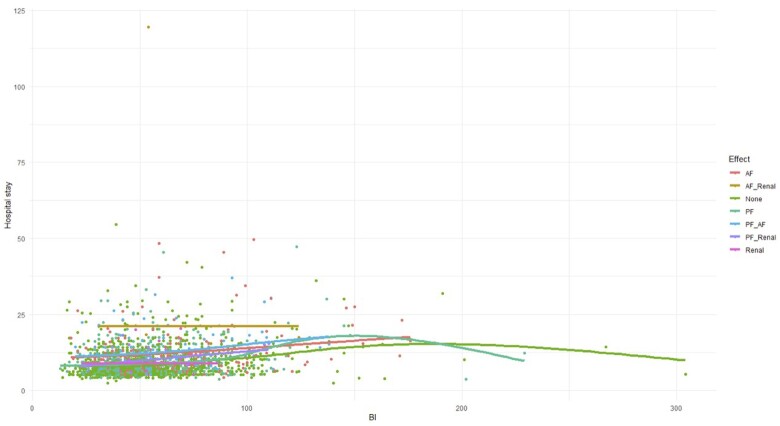
Applying the GAM revealed a significant non-linear relationship between BI and hospital LOS (Fig. 1). Higher BI was associated with an increased LOS, with diminishing returns observed at higher BI.
Figure 1:
Scatter plot of BI vs. hospital length of stay using a generalized additive model. BI: bypass index.
Secondary outcomes
At logistic regression analysis, BI was predictive of new renal dysfunction and new cardiac rhythm disturbance at univariate and multivariable regression analyses, but not pulmonary dysfunction. BI was associated with increasing ICU LOS at univariate (beta coefficient: 0.011 days per BI unit, P < 0.001) and multivariable analysis (beta coefficient 0.011 days per BI unit, P < 0.001) (Table 3).
Table 3:
Univariate and multivariable regression analysis
| Univariate OR (95% CI) | P-value | Multivariable OR (95% CI) | P-value | |||
|---|---|---|---|---|---|---|
| Any postoperative organ dysfunction | BIa | 1.005 (1.002, 1.009) | 0.001 | 1.009 (1.005, 1.012) | <0.001 | |
| Postoperative cardiac dysfunction | BIa | 1.013 (1.009, 1.017) | <0.001 | 1.014 (1.01, 1.019) | <0.001 | |
| Postoperative renal dysfunction | BIa | 1.001 (1.001, 1012) | 0.022 | 1.007 (1.001, 1.013) | 0.013 | |
|
| ||||||
| Univariate beta coefficient (95% CI) | P-value | Multivariable beta coefficient (95% CI) | P-value | |||
|
| ||||||
| ICU LOS | BIa | 0.011 (0.01, 0.012) | <0.001 | 0.011 (0.01, 0.012) | <0.001 | |
BI per unit.
OR: odds ratio; CI: confidence interval; BI: bypass index; ICU: intensive care unit; LOS: length of stay.
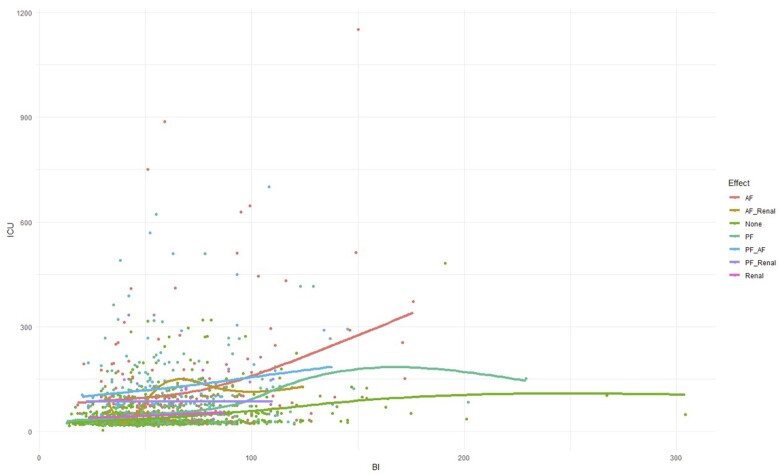
Applying GAMs showed a statistically significant non-linear relationship between BI and length of ICU stay. The most significant predictor of increased ICU LOS was cardiac dysfunction. The increase in stay continued to increase non-linearly as BI increased, without showing any diminishing returns (Fig. 2).
Figure 2:
Scatter plot of BI vs. ICU length of stay using a generalized additive model. BI: bypass index; ICU: intensive care unit.
We undertook a post hoc analysis to see if BI was more predictive of outcome than CPB time alone. At regression analysis increasing CPB time was found to be predictive of increased hospital LOS in univariate and multivariable models. Furthermore, CPB time was significantly higher in patients who developed any new organ dysfunction, new cardiac rhythm disturbance, and new renal dysfunction (Table 4).
Table 4:
Primary outcome for non-indexed CPB time
| Hospital length of stay (death within 30 days excluded) | ||||
|---|---|---|---|---|
| Univariate beta coefficient (95% CI) | P-value | Multivariable beta coefficient (95% CI) | P-value | |
| CPB time (per minute) | 0.002 (0.002, 0.003) | <0.001 | 0.019 (0.015, 0.024) | <0.001 |
CPB: cardiopulmonary bypass; CI: confidence interval.
DISCUSSION
We retrospectively studied adult patients undergoing elective or semi-urgent cardiac surgery on CPB to investigate the utility of indexing bypass time to BSA, creating a BI. Our primary outcome was postoperative LOS and we found that higher BI was associated with increased postoperative LOS in hospital as well as in ICU. Further analyses demonstrated a positive association between increasing BI and risk of new renal dysfunction and new cardiac rhythm disturbance. On regression analyses, we found that BI possessed a similarly strong association as CPB time alone.
CPB flow is generally adjusted to BSA and proponents of more individualized approaches like goal-directed perfusion are hoping to ameliorate the negative effects of extracorporeal circulation in patients although results are contradictory [19, 20]. The negative effects of CPB, however, are dose—i.e. time—dependent. We, therefore, proposed the BI as a means of more accurately quantifying bypass exposure. Similar to cardiac output measurements, where a single number without context has little meaning, normalizing time spent on CPB to BSA provides patient context. The reasons for that are 3-fold: first, there is a greater haemodilution effect in smaller patients with a lower BSA. This fact, secondly, leads to a higher risk of red blood cell transfusion and associated complications in patients with lower BSA [21, 22]. Thirdly, prior work has proposed that an ‘obesity paradox’ may be present in cardiac surgery, where patients with a higher BMI (and therefore BSA) have better outcomes after cardiac surgery involving CPB than smaller patients [17]. In a cohort of 3560 patients undergoing CABG smaller patient size correlated with increased operative mortality, myocardial infarction, cerebrovascular accident and hospital LOS [23]. Several studies have demonstrated the association between increased time on CPB and poorer clinical outcomes. Analysis of a single-centre cohort of 5006 patients demonstrated an association between increased time on CPB and several patient-centred outcomes including mortality, renal dysfunction, neurological dysfunction and pulmonary dysfunction [13]. These cohort studies have been criticized for not taking into consideration that longer CPB times are often due to higher complexity, thus increasing morbidity and mortality associated with the operation.
Our study demonstrated the risk of increased morbidity with higher BI or longer CPB time, giving credence to the suggestion that expeditious surgery is not only associated with less morbidity but also better resource utilization.
We did not observe an association between bypass and mortality, which may be explained by our low mortality rate (0.6%). Salis et al. [13] observed 2.6% mortality in their cohort, the higher event rate allowed the association between duration of CPB and mortality. In view of the low event rate, we chose to use LOS as a surrogate marker for morbidity as our primary outcome rather than mortality. An analysis of administrative data from the Global Comparators Project, including over 4 million admissions, found that patients in the upper quartile of LOS had higher odds of mortality (OR: 1.45, 95% CI: 1.43–1.47) and a higher morbidity burden [24]. Postoperative LOS is patient-centred, institution-centred and population-centred. LOS may be influenced by confounding factors, however, at our institution discharge criteria are protocolized limiting the likelihood of this. To keep data as homogeneous as possible we restricted data analysis to operations before the first wave of SARS-CoV-2 started compromising healthcare in the UK, particularly hospital discharge of medically fit patients, which is still relevant to this day.
The BI of patients with a hospital stay ≥ median time in our cohort is significantly higher than that of patients with a shorter stay.
A multitude of risk factors for postoperative atrial fibrillation (AF) have been described over the years. Besides hypokalaemia [25] and hypomagnesaemia [26], no clear intraoperative risk factors have been identified. To the best of our knowledge, only one study investigated a possible association between duration of CPB and aortic cross-clamping and postoperative AF but yielded inconclusive results [27]. In our study, there was a significant association between above mean BI or CPB time and postoperative AF in all included patients and in the CABG-only group.
CPB-associated renal dysfunction accounts for the biggest burden of morbidity after cardiac surgery and is associated with a more than 2-fold increase in early mortality regardless of the definition used for acute kidney injury (AKI) [28]. The global incidence of AKI after cardiac surgery is 22.3% across all accepted definitions [29]. A single-centre review of 3575 patients in a German University Hospital showed that fewer patients develop AKI if surgery and ischaemic time is kept short, blood loss is kept to a minimum and CPB is conducted in normothermia [12]. A review of over 11 000 case records in Italy showed that time spent on CPB was associated with an increased risk in renal failure requiring renal replacement therapy. The statistical significance was lost after adjusting for confounders [30]. The results from our study corroborate earlier findings that longer CPB time is associated with an increased incidence of renal dysfunction.
In addition to ischaemia-reperfusion injury, the cause of postoperative pulmonary dysfunction is likely to be linked to anatomical and physiological factors Firstly, the lungs act as a filter in the venous circulation and therefore all active and activating substances generated during CPB will transit through them; secondly, the smaller lung capillaries are more prone to trapping debris and aggregates, which in turn leads to higher local activation of inflammatory mediators; thirdly, the lungs are home to a considerable pool of neutrophils. We did not see an association between BI and pulmonary dysfunction in our cohort, neither overall nor in the isolated CABG group. This might be explained by the fact that the triggers are an exposure effect rather than dose-dependent. Earlier studies exploring lung perfusion with protective solutions like Celsior during CPB and aortic cross-clamping have not led to improved postoperative lung function [31]. The fact that pulmonary function is equally not influenced by the inspired oxygen fraction of gas insufflated from the ventilator during aortic cross-clamping [32] might underpin the theory that ischaemia-reperfusion injury in addition to an overwhelming burden of inflammatory metabolites flooding the lung precipitate pulmonary dysfunction regardless of duration of CPB.
Limitations
Our study has several limitations. First, we are not able to report on neurological outcomes such as postoperative delirium or confusion, which are known to increase ICU and postoperative LOS [33]. Since the group with a LOS above median stay has a significantly higher BI it is possible that neurological complications are a contributor to the increased LOS. We do not have the data for potential confounders, such as pre-existing dementia, but think that an additional study might be warranted.
Second, due to its retrospective nature, there is the possibility that missing or incorrect data may have influenced our results. Of 2413 eligible patients, only 119 were excluded due to incomplete data. CPB and patient demographic data are directly imputed on the day of surgery into the locally curated CARDS II database. Outcome variables were directly retrieved from the ICU electronic record-keeping system, and our outcome variables of interest were specifically selected to minimize the risk of data error. Despite this, it is conceivable that missing data may have impacted our results.
Third, this is a single-centre study and so may lack external validity. Our initial intention was to undertake a multicentre study. However, when other centres were approached, we encountered issues with data access, and they could not vouch for the completeness of their data sets. To preserve the integrity of our study, we decided to use a single, well-maintained dataset from a digitally mature institution so as not to negatively impact the validity of our findings.
Fourth, it is possible that the CPB time component of BI is confounded by other factors such as surgical complexity, or patient comorbidity. It remains plausible that unrecognized confounding factors may have influenced our results.
Fifth, we used a relatively short data collection period of 23 months. Data in the two large cohort studies was collected over 6 years, but it is possible that cardiac surgical, anaesthesia and CPB practice and equipment may have changed over this period, thereby possibly confounding results. Using a shorter inclusion period will have limited this effect.
Despite the above limitations, the study shows that reducing bypass time is likely to be associated with reduced LOS, better outcomes and less morbidity. It can, of course, be argued that long bypass times are dictated by the technical difficulty of a particular procedure, so that this factor alone is responsible for the adverse outcomes. This is true to some extent, but bypass times are also affected by the speed of individual surgeons, the choice of surgical technique and the addition of procedures the indications for which are borderline. In surgery generally, and cardiac surgery especially, speed is good provided no corners are cut and the final technical result is not compromised. Our study should raise awareness that unnecessary delay in the completion of the surgical procedure may have an adverse effect on outcomes and should be avoided where possible. When the delay is due to the patient, little can be done. When it is due to the surgeon, there may be room for improvement.
CONCLUSION
In our cohort of 2294 patients undergoing cardiac surgery requiring CPB at an academic, quaternary referral cardiothoracic hospital, we tested a novel index of bypass exposure, the BI. Although we demonstrated that indexing CPB time to BSA was associated with postoperative LOS, ICU LOS, new renal injury and new cardiac rhythm disturbance, it did not appear to have a stronger association with these outcome measures than bypass time alone.
Supplementary Material
ACKNOWLEDGEMENTS
None.
Glossary
ABBREVIATIONS
- AKI
Acute kidney injury
- BI
Bypass index
- BMI
Body mass index
- BSA
Body surface area
- CPB
Cardiopulmonary bypass
- GAM
Generalized additive model
- ICU
Intensive care unit
- IQR
Interquartile range
- LOS
Length of stay
- SD
Standard deviation
Contributor Information
Florian Falter, Department of Anaesthesia and Intensive Care, Royal Papworth Hospital, Cambridge, UK.
Ryan Salter, Department of Anaesthesia and Intensive Care, Wellington Hospital, Wellington, New Zealand.
Jose Fernandes, Department of Clinical Perfusion, Royal Papworth Hospital, Cambridge, UK.
Christiana Burt, Department of Anaesthesia and Intensive Care, Royal Papworth Hospital, Cambridge, UK.
Kate Drummond, Department of Anaesthesia, Royal Adelaide Hospital, Adelaide, Australia.
Ganesh Ramalingam, Department of Anaesthesia and Intensive Care, Royal Papworth Hospital, Cambridge, UK.
Samer Nashef, Department of Cardiothoracic Surgery, Royal Papworth Hospital, Cambridge, UK.
SUPPLEMENTARY MATERIAL
Supplementary material is available at ICVTS online.
FUNDING
None declared.
Conflict of interest: Florian Falter served as speaker bureau for Abbott Point of Care, received consulting fees from Abbott Diabetes, and participated in advisory board for Werfen. All other authors have declared no conflict of interest.
ETHICS STATEMENT
The study was granted IRB approval (approval no. S02788) by the Royal Papworth Hospital R&D Committee, which waived informed consent due to the retrospective nature of the study.
DATA AVAILABILITY
The data supporting this study’s findings are available from the corresponding author upon reasonable request. All data are held on a secure repository within the hospital network and can be made available in anonymized form.
Author contributions
Florian Falter: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Supervision; Writing—original draft; Writing—review & editing. Ryan Salter: Data curation; Formal analysis; Writing—original draft; Writing—review & editing. Jose Fernandes: Investigation; Project administration; Writing—original draft. Christiana Burt: Conceptualization; Data curation; Investigation; Writing—original draft; Writing—review & editing. Kate Drummond: Data curation; Investigation; Writing—original draft. Ganesh Ramalingam: Data curation; Investigation; Writing—original draft. Samer Nashef: Methodology: Writing—original draft, reviewing, editing.
Reviewer information
Reviewer information Interactive CardioVascular and Thoracic Surgery thanks Tomislav Kopjar and the other anonymous reviewers for their contribution to the peer review process of this article.
References
- 1. Dancy CM, Townsend ER, Boylett A, Chan SL, Parker-Williams EJ, Parker DJ.. Pulmonary dysfunction associated with cardiopulmonary bypass: a comparison of bubble and membrane oxygenators. Circulation 1981;64:II54–7. [PubMed] [Google Scholar]
- 2. Westaby S. Organ dysfunction after cardiopulmonary bypass. A systemic inflammatory reaction initiated by the extracorporeal circuit. Intensive Care Med 1987;13:89–95. [DOI] [PubMed] [Google Scholar]
- 3. Murphy GJ, Angelini GD.. Side effects of cardiopulmonary bypass: what is the reality? J Card Surg 2004;19:481–8. [DOI] [PubMed] [Google Scholar]
- 4. Ignjatovic V, Chandramouli A, Than J, Summerhayes R, Newall F, Horton S. et al. Plasmin generation and fibrinolysis in pediatric patients undergoing cardiopulmonary bypass surgery. Pediatr Cardiol 2012;33:280–5. [DOI] [PubMed] [Google Scholar]
- 5. Weerasinghe A, Taylor KM.. The platelet in cardiopulmonary bypass. Ann Thorac Surg 1998;66:2145–52. [DOI] [PubMed] [Google Scholar]
- 6. Belhaj A. Actual knowledge of systemic inflammation reaction during cardiopulmonary bypass. Recent Pat Cardiovasc Drug Discov 2012;7:165–9. [DOI] [PubMed] [Google Scholar]
- 7. Day JR, Taylor KM.. The systemic inflammatory response syndrome and cardiopulmonary bypass. Int J Surg 2005;3:129–40. [DOI] [PubMed] [Google Scholar]
- 8. Ohata T, Mitsuno M, Yamamura M, Tanaka H, Kobayashi Y, Ryomoto M. et al. Beneficial effects of mini-cardiopulmonary bypass on hemostasis in coronary artery bypass grafting: analysis of inflammatory response and hemodilution. ASAIO J 2008;54:207–9. [DOI] [PubMed] [Google Scholar]
- 9. Rubens FD, Boodhwani M, Mesana T, Wozny D, Wells G, Nathan HJ; Cardiotomy Investigators. The cardiotomy trial: a randomized, double-blind study to assess the effect of processing of shed blood during cardiopulmonary bypass on transfusion and neurocognitive function. Circulation 2007;116:I89–97. [DOI] [PubMed] [Google Scholar]
- 10. Nadeem R, Agarwal S, Jawed S, Yasser A, Altahmody K.. Impact of cardiopulmonary bypass time on postoperative duration of mechanical ventilation in patients undergoing cardiovascular surgeries: a systemic review and regression of metadata. Cureus 2019;11:e6088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gummert JF, Barten MJ, Hans C, Kluge M, Doll N, Walther T. et al. Mediastinitis and cardiac surgery—an updated risk factor analysis in 10,373 consecutive adult patients. Thorac Cardiovasc Surg 2002;50:87–91. [DOI] [PubMed] [Google Scholar]
- 12. Wittlinger T, Maus M, Kutschka I, Baraki H, Friedrich MG.. Risk assessment of acute kidney injury following cardiopulmonary bypass. J Cardiothorac Surg 2021;16:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Salis S, Mazzanti VV, Merli G, Salvi L, Tedesco CC, Veglia F. et al. Cardiopulmonary bypass duration is an independent predictor of morbidity and mortality after cardiac surgery. J Cardiothorac Vasc Anesth 2008;22:814–22. [DOI] [PubMed] [Google Scholar]
- 14. Madhavan S, Chan SP, Tan WC, Eng J, Li B, Luo HD. et al. Cardiopulmonary bypass time: every minute counts. J Cardiovasc Surg (Torino) 2018;59:274–81. [DOI] [PubMed] [Google Scholar]
- 15. Pappalardo F, Corno C, Franco A, Giardina G, Scandroglio AM, Landoni G. et al. Reduction of hemodilution in small adults undergoing open heart surgery: a prospective, randomized trial. Perfusion 2007;22:317–22. [DOI] [PubMed] [Google Scholar]
- 16. Campbell JA, Holt DW, Shostrom VK, Durham SJ.. Influence of intraoperative fluid volume on cardiopulmonary bypass hematocrit and blood transfusions in coronary artery bypass surgery. J Extra Corpor Technol 2008;40:99–108. [PMC free article] [PubMed] [Google Scholar]
- 17. Stamou SC, Nussbaum M, Stiegel RM, Reames MK, Skipper ER, Robicsek F. et al. Effect of body mass index on outcomes after cardiac surgery: is there an obesity paradox? Ann Thorac Surg 2011;91:42–7. [DOI] [PubMed] [Google Scholar]
- 18. von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg 2014;12:1495–9.25046131 [Google Scholar]
- 19. Broadwin M, Palmeri M, Kelting T, Groom R, Robich M, Lucas FL. et al. Goal directed perfusion is not associated with a decrease in acute kidney injury in patients predicted to be at high risk for acute renal failure after cardiac surgery. J Extra Corpor Technol 2022;54:128–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ranucci M, Johnson I, Willcox T, Baker RA, Boer C, Baumann A. et al. Goal-directed perfusion to reduce acute kidney injury: a randomized trial. J Thorac Cardiovasc Surg 2018;156:1918–27e2. [DOI] [PubMed] [Google Scholar]
- 21. Galas FR, Almeida JP, Fukushima JT, Osawa EA, Nakamura RE, Silva CM. et al. Blood transfusion in cardiac surgery is a risk factor for increased hospital length of stay in adult patients. J Cardiothorac Surg 2013;8:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Engoren MC, Habib RH, Zacharias A, Schwann TA, Riordan CJ, Durham SJ.. Effect of blood transfusion on long-term survival after cardiac operation. Ann Thorac Surg 2002;74:1180–6. [DOI] [PubMed] [Google Scholar]
- 23. Schwann TA, Habib RH, Zacharias A, Parenteau GL, Riordan CJ, Durham SJ. et al. Effects of body size on operative, intermediate, and long-term outcomes after coronary artery bypass operation. Ann Thorac Surg 2001;71:521–30. discussion 30–1. [DOI] [PubMed] [Google Scholar]
- 24. Lingsma HF, Bottle A, Middleton S, Kievit J, Steyerberg EW, Marang-van de Mheen PJ.. Evaluation of hospital outcomes: the relation between length-of-stay, readmission, and mortality in a large international administrative database. BMC Health Serv Res 2018;18:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wahr JA, Parks R, Boisvert D, Comunale M, Fabian J, Ramsay J. et al. Preoperative serum potassium levels and perioperative outcomes in cardiac surgery patients. Multicenter Study of Perioperative Ischemia Research Group. JAMA 1999;281:2203–10. [DOI] [PubMed] [Google Scholar]
- 26. England MR, Gordon G, Salem M, Chernow B.. Magnesium administration and dysrhythmias after cardiac surgery. A placebo-controlled, double-blind, randomized trial. JAMA 1992;268:2395–402. [PubMed] [Google Scholar]
- 27. Aranki SF, Shaw DP, Adams DH, Rizzo RJ, Couper GS, VanderVliet M. et al. Predictors of atrial fibrillation after coronary artery surgery. Current trends and impact on hospital resources. Circulation 1996;94:390–7. [DOI] [PubMed] [Google Scholar]
- 28. Pickering JW, James MT, Palmer SC.. Acute kidney injury and prognosis after cardiopulmonary bypass: a meta-analysis of cohort studies. Am J Kidney Dis 2015;65:283–93. [DOI] [PubMed] [Google Scholar]
- 29. Hu J, Chen R, Liu S, Yu X, Zou J, Ding X.. Global incidence and outcomes of adult patients with acute kidney injury after cardiac surgery: a systematic review and meta-analysis. J Cardiothorac Vasc Anesth 2016;30:82–9. [DOI] [PubMed] [Google Scholar]
- 30. Mancini E, Caramelli F, Ranucci M, Sangiorgi D, Reggiani LB, Frascaroli G. et al. Is time on cardiopulmonary bypass during cardiac surgery associated with acute kidney injury requiring dialysis? Hemodial Int 2012;16:252–8. [DOI] [PubMed] [Google Scholar]
- 31. Buggeskov KB, Maltesen RG, Rasmussen BS, Hanifa MA, Lund MAV, Wimmer R. et al. Lung protection strategies during cardiopulmonary bypass affect the composition of blood electrolytes and metabolites-a randomized controlled trial. J Clin Med 2018. Nov 21;7(11):462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Salter R, Parmar J, Alyward L, McKie MA, Falter F.. Association of passive lung insufflation oxygen fraction in adult patients on cardiopulmonary bypass with postoperative pulmonary outcomes: a retrospective cohort study. J Cardiothorac Vasc Anesth 2022;36:461–8. [DOI] [PubMed] [Google Scholar]
- 33. Rudolph JL, Marcantonio ER.. Review articles: postoperative delirium: acute change with long-term implications. Anesth Analg 2011;112:1202–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting this study’s findings are available from the corresponding author upon reasonable request. All data are held on a secure repository within the hospital network and can be made available in anonymized form.