Abstract
Malignant (high-grade) gliomas are aggressive intrinsic brain tumors that diffusely infiltrate the brain parenchyma. They comprise of World Health Organization (WHO) grade III and IV gliomas. Ionizing radiation or irradiation (IR) is frequently utilized in the treatment of both primary as well as metastatic brain tumors. On the contrary, macrophages (MΦ) are the most copious infiltrating immune cells of all the different cell types colonizing glioma. MΦ at tumor milieu are referred to as tumor-associated macrophages (TAMΦ). In malignant gliomas milieu, TAMΦ are also polarized into two distinct phenotypes such as M1 TAMΦ or M2 TAMΦ, which are capable of inhibiting or promoting tumor growth, respectively. Cranial-IR such as x- and γ-IR are sufficient to induce the migration of peripherally derived MΦ into the brain parenchyma. The IR facilitate a more immunosuppressive milieu via the stimulation of efferocytosis in TAMΦ, and an upsurge of tumor cell engulfment by TAMΦ exhibited detrimental effect of the anti-tumoral immune response in glioma. The MΦ inside the tumor mass are associated with multiple phenomena that include IR resistance and enrichment of the M2 MΦ after IR is able to facilitate glioblastoma multiforme (GBM) recurrence. Reviews on the role of cranial IR-induced peripheral and brain-engrafting macrophages (BeMΦ) in glioma are lacking. Specifically, most studies on peripheral, intrinsic as well as beMΦ on IR focus on WHO grade III and IV. Thus, this review precisely focuses primary on WHO grade III as well as IV gliomas.
Keywords: Irradiation, glioma, malignant, macrophages, radiosensitization, radioresistance
Introduction
Malignant (high-grade) gliomas are aggressive intrinsic brain tumors that diffusely infiltrate the brain parenchyma.1-4 They are rapidly progressive comprising of World Health Organization (WHO) grade III that includes anaplastic astrocytoma, anaplastic oligodendroglioma, mixed anaplastic oligoastrocytoma, and WHO grade IV, also referred to as glioblastoma multiforme (GBM).2,3,5 The incidence of high grade is about 5 out of 100,000 and they are made up of 35% to 45% of all primary brain tumors.1,6 Notably, anaplastic astrocytomas constitute about 10% to 15%, while anaplastic oligodendrogliomas as well as anaplastic oligoastrocytomas constitute about 10% of all malignant gliomas.3,6
The GBM is mostly common in adults and it is still the most challenging brain tumor clinically.7-9 Epidemiologically, GBM constitutes about 14.5% of all central nervous system (CNS) tumors and 48.6% of malignant CNS tumors with a median overall survival as low as 15 months.7,10 Pathologically, it is an extremely heterogeneous tumor with distinct co-existing cell categories such as tumor cells, fibroblasts, endothelial cells, as well as diverse immune cells. 11 Standard treatments modalities comprise of utmost safe surgery followed by external radiotherapy as well as simultaneous chemotherapy.12,13 Notably, current mechanical and treatment scheme targets the tumor cells and neglects other cellular components such as macrophages (MΦ) recruited to the GBM or tumor milieu.
Ionizing radiation or irradiation (IR) is frequently utilized in the treatment of both primary as well as metastatic brain tumors.14,15 Notably, whole-brain radiotherapy (WBRT), delivered in multiple fractions, is routinely utilized for the treatment of patients with both primary as well as metastatic brain tumors.14,15 On the contrary, MΦ are the most copious infiltrating immune cells of all the different cell types colonizing GBM. Notably, MΦ at tumor milieu are referred to as tumor-associated macrophages (TAMΦ). 16 Interestingly, circulating monocytes are capable of migrating to the tumor milieu and once in tumor milieu, they segregate into MΦ (M0 MΦ) via the stimulation cytokines. 17 Intriguingly, M0 MΦ show a high level of plasticity and are polarizable into two distinct functional phenotypes such as M1 MΦ and M2 MΦ once differentiated. 18
In GBM milieu, TAMΦ are also polarized into two distinct phenotypes such as M1 TAMΦ or M2 TAMΦ, which are capable of inhibiting or promoting tumor growth, respectively.19-21 Notably, M1 TAMΦ are proinflammatory and they generate high quantities of inducible nitric oxide synthase (iNOS) whereas M2 TAMΦs are anti-inflammatory, pro-angiogenic, accelerates metastasis, and generate high quantities of Arg I. 21 Remarkably, primary TAMΦ function as M1, but are progressively changed to M2 as the tumor advances. 22 Notably, M1 and M2 TAMΦ dominate in distinctive areas of the tumor milieu, with M2 TAMΦ migrating and accumulating in avascular and hypoxia regions.19,23 Thus, M1/M2 TAMΦ ratio may vary in different parts of the tumor.
In GBM microenvironment, TAMΦs are very crucial during the recurrence of GBM and their presence signifies tumor aggressiveness as well as of the overall survival of those patients with GBM. 24 Interestingly, WBRT causes detrimental effects to the CNS milieu leading to the accumulation of peripherally derived MΦ.14,25 However, reviews on the pivotal role of cranial IR-induced peripheral and brain-engrafting macrophages (BeMΦ) in glioma are lacking. Specifically, most studies on peripheral, intrinsic, as well as beMΦ on IR focus on WHO grade III and IV. Thus, this review precisely focuses primarily on WHO grade III as well as IV gliomas.
Literature Search Method and Scope of Review
The “Boolean logic” was used to search for article on the subject matter in PubMed and PubMed central as well as google scholar with search terms such as cranial IR and/or peripheral, intrinsic, beMΦ and/or glioma. Thus, this review discuses in vivo as well as in vitro studies that describe the association between cranial IR and peripheral, intrinsic, beMΦ in glioma at the bench level. Studies on microglia were excluded because this review focuses primarily on the effect of cranial IR on peripheral, intrinsic, as well as beMΦ in glioma. However, to clearly differentiate between microglia and other MΦ, articles that differentiate between the two were discussed. Also, articles that discuss MΦ and IR-induced abscopal and bystander effects were discussed.
Notably, articles that define or describe specific factors but are not related to cranial IR and peripheral, intrinsic, beMΦ in glioma were acknowledged and cited accordingly. Importantly, articles that describe the association between cranial IR and peripheral, intrinsic, beMΦ in glioma are discussed in subheadings such as, (1) residual MΦ, microglia, and BeMΦ, (2) MΦ at Tumor and IR Tumor Milieu, (3) IR and MΦ subtypes, (4) IR-induced hypoxia and MΦ signaling pathways, (5) MΦ induce radiosensitization and radioresistance, and (6) MΦ and IR-induced abscopal and bystander effects in accordance to how research is conducted at bench level. Conclusions and perspectives were drawn from the subheading above.
Residual MΦ, Microglia, and BeMΦ
Residual brain MΦ are characterized into perivascular, meningeal, circumventricular organs, and choroid plexus MΦ based their anatomical locations.19,26 Also, all residual brain MΦ are located in the perivascular or Virchow-Robin spaces, subdural meninges, whereas choroid plexus originated from short-lived blood monocytes after birth, which are rapidly replaced by bone marrow derived cells.19,26,27 Furthermore, perivascular and meningeal MΦ are produced from embryonic yolk sac precursors, whereas choroid plexus MΦ have dual embryonic and adult haematopoietic origins. 26 Moreover, residual brain MΦ are often restricted at the boundary between the parenchyma and the circulation. 19
Microglia are the original phagocytes of the brain as well as spinal cord and they are heterogeneously situated in almost all non-overlapping sections of the brain and the spinal cord.28,29 Notably, they are responsible for the detection and engulfing of extracellular components like cell debris, apoptotic cells, tumors cells and microbes.19,28 Also, microglia usually communicate with neuronal circuits in developing and in the adult brain.29,30 Similarly, microglia stimulate neuronal apoptosis, eliminate less functional synaptic connections like synaptic trimming and induce neuronal activity.31,32 It was observed that in GBMs, TAMΦ were not brain-resident microglia, but mainly monocyte-derived MΦ from peripheral blood in GBM milieu. 33
It is worth noting that obvious differences in functions, abundance, as well as spatial distribution have been identified between beMΦ and microglia. 34 In addition, beMΦ such as monocyte-derived MΦ and TAMΦ as well as microglia have been investigated separately and their distinct functions in GBM pathogenesis observed using single-cell omics and fate-mapping systems. 35 Furthermore, human GBM was mainly infiltrated by monocyte-derived MΦ rather than microglia during a single-cell immunohistochemical analysis. 36 Moreover, beMΦ were spatially distributed in the center of the tumor while microglia were spatially distributed at the invasive margin. 34
Also, beMΦ were capable of expressing more pro-inflammatory factors than microglia during single-cell and bulk gene expression data analysis. 37 Notably, beMΦ signatures such as gene expression and epigenetic marks were precipitously lost upon ex vivo culture, as were best recognized in microglia. 38 Similarly, beMΦ expressed genes such as C-C motif chemokine receptor 2 (CCR2), interferon alpha and beta receptor subunit 1(IFNAR1), membrane Spanning 4-domains A7 (MSA4A7) and apolipoprotein E (Apo-E) that were absent from host microglia, and exhibited MΦ scavenger receptor 1 (MSR1) as well as anexelekto (AXL) mRNAs that correlated with the absence of spalt-like transcription factor 1 (SALL1). 39 Moreover, transcriptomes analysis of beMΦ revealed similarity with perivascular MΦ and deficiency of a regulatory pathway stimulated by interleukin (IL)-10, as compared to host microglia. 40
Colony-stimulating factor 1 receptor (CSF1R) is a receptor tyrosine kinase responsible for development as well as functions of myeloid cells such as monocytes and MΦ.41,42 Comparative transcriptome analysis revealed that beMΦ in mice tentatively depleted microglia due to a CSF1R deficiency maintaining a transcriptional identity different from host cells. 43 Also, selective depletion of resident microglia without blood–brain barrier (BBB) disruption triggered a permissive environment for the recruitment, infiltration, as well as the engraftment of peripheral MΦ.14,44 Thus, peripherally derived MΦ were capable of spatially replacing microglia as well as developed ramifications analogous to microglia. 45
It is worth noting that beMΦ sustain their distinctive transcriptional as well as functional uniqueness in three different beMΦ engraftment models. 43 Also, persistent loss of microglia as well as their failure to repopulate the niche was adequate to stimulate beMΦ engraftment into the brain and spinal cord in the absence of IR. 43 Furthermore, beMΦ were able to replace microglia only when microglia are compromised in their capability to repopulate the niche, without the need for IR, inflammation, or BBB disruption. 43 Moreover, spatial distribution of microglia and MΦ stimulated differential GBM cells and their viability to phagocytes after IR exposure. 43
MΦ at Tumor and IR Tumor Milieu
Gliomas are histologically classified into Grades I to IV according to the WHO criteria.46,47 Also, based on histological classification above, Grade III comprises astrocytoma or anaplastic astrocytoma, whereas GBM forms Grade IV gliomas. 48 Furthermore, GBM are also further classified into isocitrate dehydrogenase (IDH)-wild type, IDH-mutant, not-otherwise-specified and not-elsewhere-classified.49,50 Similarly, the IDH-wild type constitutes about 90% of cases and it de novo starts at about 60 years of age, whereas the IDH-mutant constitutes about 10% of cases and is often a secondary GBM.49,50
These secondary GBM usually develop in younger patients with gliomas of higher differentiation such as WHO Grades I to III.50,51 It is worth noting that IDH-mutant carries an expressively better prognosis than wild type IDH. 51 Also, in not-otherwise-specified type, the IDH mutation status is often not determined because of lack of histological or molecular material for testing, whereas the not-elsewhere-classified is the fourth type that was identified in recent years.49,50 Thus, most studies on peripheral, intrinsic, as well as beMΦ on IR in glioma focus on grades III and IV.
Notably, specific molecular subtypes of gliomas are capable of influencing the abundance as well as functional characteristics of MΦ. 35 Also, grade IV or GBM tend to contain a higher abundance of TAMΦ than other subtypes of glioma. 52 Similarly, a single-cell RNA sequencing (scRNA-seq) analysis has shown that recurrent GBM has more infiltrating TAMΦ than primary GBM. 53 Moreover, higher MΦ enrichment was detected in the mesenchymal subtype of GBM than in the proneural as well as classical subtypes. 54 Furthermore, the difference above was due to the NF1 mutation typically seen in mesenchymal GBM as NF1 was capable of modulating myeloid cell chemotaxis. 54
In addition, stimulated MΦ were capable of triggering the secretion of proteolytic enzymes as well as inflammatory cytokines. 55 Also, MΦ, which are inflammatory cells in GBM, facilitated tumor development as well as symbolized a negative prognostic factor.16,56 It is worth noting that mutations in IDH genes were capable of influencing MΦ infiltration in GBM. Precisely, IDH wild type had a higher level of infiltrating MΦ compared to IDH-mutant GBM during scRNA-seq analysis. 35 Similarly, better prognosis was observed in IDH-mutant GBM patients compared to IDH-wild type patients GBM. 35
Notably, TAMΦ are recruited during the early stage of GBM tumorigenesis and they are mostly located in the perivascular regions. 57 In GBM milieu, TAMΦ expressed higher concentration of major histocompatibility complex (MHC)-II but could not interact with T cells localized in the tumor periphery.35,58 Furthermore, negative correlation between TAMΦ infiltration and survival was observed in adult patients with GBM. 59 However, contradictory study revealed positive correlation between CD68+ CD163+ CD206+ TAMΦ infiltration and the overall survival of patients with IDH1R132H-wild type GBM. 60
Interestingly, immunostaining of MΦ and small vessels in resected glioma specimens revealed an augmented numbers of infiltrating MΦ and small vessel density in GBM compared to astrocytomas or anaplastic astrocytomas. 61 Also, MΦ infiltration correlated with vascular density in human gliomas and heme oxygenase-1 (HO-1), a rate-limiting enzyme in heme catabolism, was also linked to the stimulation of MΦ. 61 Moreover, secretion of mRNA encoding HO-1 correlated with MΦ infiltration as well as vascular density in human glioma. 61 In addition, infiltrating MΦ were positively stained with anti-HO-1 antibody via immunohistochemical analysis, as well as in situ hybridization for HO-1 revealed that HO-1 was secreted in infiltrating MΦ in gliomas. 61 Thus, HO-1 gene is a promising marker for MΦ infiltration as well as neovascularization in human gliomas.
Cranial-IR such as X- and γ-IR are sufficient to induce the migration of peripherally derived MΦ into the brain parenchyma.14,62 Also, an acute response to IR exposure was responsible for the induced peripheral MΦ immigration into the brain in seven-day time. 14 It is worth noting that IR was capable of influencing the tropism of MΦ in the tumor by augmenting the generation of chemokines at the origin of MΦ migration.14,62 It is observed that beMΦ underwent clonal proliferation and thereby likely progressively outcompeted IR.14,62 Also, tissue MΦ such as Kupffer cells and alveolar MΦ were capable of replacing by bone marrow-derived cells in IR chimeras and other small animal models deficient of resident MΦ. 63 Ionized calcium binding adaptor molecule 1 (Iba1) is a MΦ-specific calcium-binding protein. 64
It is worth noting that beMΦ enter the brain 14 days after the completion of brain IR, or 4 days after the CSF-1Ri withdrawal. 25 Moreover, the ratio of beMΦ was high at 14 days after the completion of brain IR but with no restoration of the total number of Iba1 positive cells. 25 Furthermore, stromal cell-derived factor 1 (SDF-1) is a member of the CXC group of chemokines (CXCL12) and it is an endogenous ligand for the chemokine receptor CXCR4. In addition, IR facilitated the recruitment of MΦ in GBM practically 20 days post-IR by augmenting the SDF-1 production.65,66 Moreover, the early response after IR in high-grade glioma was depicted with astrocytic gliosis, vascular proliferation, and infiltration of MΦ. 67
IR and MΦ Subtypes
It is worth noting that MΦ are also capable of polarizing into two distinct functional phenotypes such as M1 MΦ and M2 MΦ once differentiated following IR exposure. 62 MΦ described herein are beMΦ. Distinctly, x-IR exposure triggered a local reoxygenation resulting in modulation of MΦ phenotype.68,69 Also, while IR was capable of augmenting M1 MΦ markers in earlier study, 70 a later study failed to detect any alteration in cytokine production. 71 Similarly, augmentation in M2 MΦ markers was detected in GBM in in vivo models of MΦ following exposure to IR. 20 Furthermore, x-IR exposure triggered a loss of MΦ present in GBM and the percentage of M2 MΦ such as CD206+ cells relative to total MΦ such as CD68+ cells was increased after IR. 62
Interestingly, CD68+ cells were detected outside the tumor core signifying that CD68+ cells were recruited within the GBM.66,72 Also, an upsurge in M2 MΦ population selectively triggered cell death of M0 as well as M1 MΦ and compared to M0 as well as M1 MΦ, M2 MΦ were less sensitive to IR during in vitro experiments. 62 In addition, M0 and M1 MΦ were capable of repairing DNA DSBs although their proportion were decreased post-IR. 62 Thus, M0 and M1 MΦ misrepair DNA DSBs resulted in certain genomic instabilities. Moreover, x-IR was also capable of augmenting M2 MΦ percentage in a recurrent GBM model. 62 It is worth noting that an upsurge in MΦ migration, which was parallel to an upsurge in M2 MΦ quantity, was detected after 22 days post-IR at which time MΦ were recruited in GBM.20,66
Similarly, an upsurge in the quantity of M2 MΦ, which was the phenotype relatively resistant to IR, was detected before MΦ recruitment. Interestingly, M0 and M1 MΦ were more sensitive to IR than M2 MΦ. 62 Also, augmented secretion of p-extracellular signal-regulated kinase (p-ERK) and p-protein kinase B (p-AKT) were detected in M2 MΦ relative to M0 and M1 MΦ implying that M2 MΦ are more radioresistant. 62 Notably, p-ERK/pan-ERK as well as p-AKT/pan-AKT are recognized as two major players in radioresistance. 62 Also, M0 and M1 MΦ are usually located in oxygenated areas of GBM following IR. 68 Thus, a significant reduction in the number of M0 and M1 MΦ was observed in 20% O2 in vitro GBM model following IR.
In addition, M0 MΦ quantity remained stable after IR whereas M1 MΦ were still reduced in 0.2% O2, which is considered as severe hypoxia. 62 Moreover, decrease in DNA DSBs was observed in M0 MΦ at 0.2% O2. This signifies that at low O2 pressure, M0 MΦ were already programed toward an M2 MΦ. 68 Similarly, M1 MΦ produced significant concentrations of NO, which decreased hypoxia. 68 However, NO generated M1 MΦ was still superior to the quantity generated in M0 and M2 MΦ. 62 Also, M2 MΦ were capable of repairing DNA DSBs and were more radioresistant to x-IR. 62 Moreover, cell death was inhibited by M2 MΦ in hypoxic conditions thus allowing M2 MΦ to be formed at detriment of M0 and M1 MΦ. 68 Furthermore, M2 MΦ were associated to glioma stem cells (GSCs) in hypoxic areas and facilitated tumor development. 33
IR Induce Hypoxia and MΦ Signaling Pathways
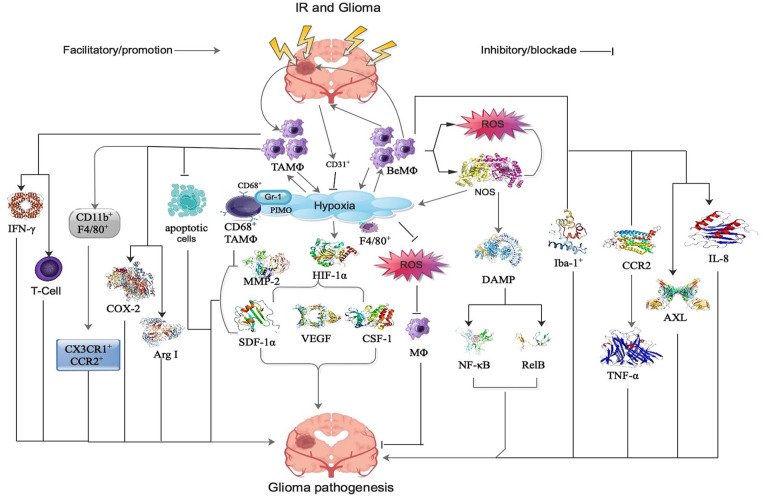
In GBM milieu, the most common non-neoplastic cells are TAMΦ, which are made up of peripheral MΦ or beMΦ, brain-intrinsic MΦ, and microglia.73,74 Notably, TAMΦ are a critical component of the local tumor milieu, and they influence tumor immune evasion, suppress T cell activity, as well as control initiation, progression and metastasis. 75 Also, TAMΦ comprise about 30% of infiltrating cells and their infiltration is authentically associated with the outcome of GBM patients. 76 Furthermore, GBM milieu is influenced by immune-related signaling pathways such as innate immune cascade, inflammatory cascade, and complement cascade activation, via TAMΦ. 73 Principally, interferon-gamma (IFN-γ) and T-cell proliferation are most influenced via TAMΦ following IR in GBM (see Figure 1). 73
Figure 1.
Shows the signaling modalities of cranial IR-induced peripheral MΦ, BeMΦ, as well as TAMΦ in the pathogenesis of glioma. Refer to the text for detailed explanations. AXL indicates anexelekto; BeMΦ, brain-engrafting macrophages; COX-2, cyclooxygenase-2; CSF, colony-stimulating factor; DAMP, damage-associated molecular pattern; HIF, hypoxia-induced factor; Iba, ionized calcium-binding adaptor; IFN, interferon-gamma; IL, interleukin; IR, ionizing radiation or irradiation or radiotherapy MMP, matrix metalloproteinase; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; PIMO, pimonidazole; ROS, reactive oxygen species; SDF, stromal cell-derived factor; TAMΦ, tumor-associated macrophages; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor.
MΦ are more resistant to IR than other cells and they react by augmenting the generation of reactive oxygen species (ROS) and NOS. 77 MΦ-induced ROS and NOS triggers the secretion of damage-associated molecular patterns (DAMP) from damaged cells, induce inflammatory transcription factors as nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) as well as RelB resulting in the secretion of inflammatory cytokines and chemokines in GBM (see Figure 1).67,77 This cascade leads to angiogenesis, edema, as well as tissue damage, but will also recruit more inflammatory cells by chemotaxis and thus alter the immune-milieu (see Figure 1).67,77 Also, hypoxia was capable of decreasing ROS accumulation and this correlated with a reduction in M0 MΦ death following IR (see Figure 1). 62
It is worth noting that hypoxic tumor milieu is mostly infiltrated by TAMΦ, which constitute the largest population of infiltrating inflammatory cells. 65 Also, the relationship between TAMΦ and hypoxia is believed to be bidirectional (see Figure 1). 20 Interestingly, IR-induced augmentation in TAMΦ in tumor core was essentially associated with the development of IR-induced chronic hypoxia. 20 Furthermore, IR-induced augmentation in TAMΦ at the tumor-invading front was triggered by mechanism other than chronic hypoxia, because this region exhibited higher microvascular density. 65
In addition, hypoxia-induced factor-1-alpha (HIF-1α) stabilization triggered the secretion of angiogenic factor such as vascular endothelial growth factor (VEGF) and chemotactic factors such as SDF-1α and CSF-1 by hypoxic tumor cells following IR (see Figure 1).20,78 Notably, these factors also recruited peripheral MΦ to the hypoxic tumor milieu to restore blood delivery as well as nurture the hypoxic cells following IR. 20 Also, it was observed that the association between TAMΦ and hypoxia was not only tumor type dependent, but also stroma dependent. 20
Interestingly, a majority of the TAMΦ in the primary tumor core were CD682+ as well as F4/802+.65 Also, TAMΦ in the invading tumor front were both CD682+ and F4/802+ as well as F4/80+ and CD68– TAMΦ following IR (see Figure 1). 79 Furthermore, the hypoxic regions in IR tumors or tumors growing in pre-IR tissues had more CD68+ TAMΦ accumulation compared to control tumors in experiments involving ALTS1C1 astrocytoma and murine GL262 tumors models. 20 Notably, pimonidazole (PIMO) is a hypoxia marker for the detection of glioma aggressiveness and metastasis.
It is worth noting that CD68+, but not F4/80+ induced TAMΦ selectively amass in PIMO+ hypoxia regions in intracranial ALTS1C1 astrocytoma following IR (see Figure 1). 20 Therefore, the association of CD68+ TAMΦ with hypoxia is tumor dependent. Contrarily, in murine GL261 brain glioma models, CD68+ TAMΦ did not have preference for PIMO+ hypoxic regions. 20 Also, most PIMO+ hypoxic regions in control tumors composed of CD31+ vessels, signifying that abnormal vessel perfusion triggered hypoxia and this led to transient hypoxia. 20 Contrarily, most IR-induced hypoxic regions did not contain CD31+ vessels, signifying that the hypoxia was triggered by insufficient blood vessels and this resulted in avascular chronic hypoxia (see Figure 1). 20
The IR-induced hypoxic regions typically develop central necrosis resulting in the accumulation of positive gamma response 1 (Gr-1+) neutrophils. 20 The Gr-1 is a marker for granulocytes. Also, pre- and post-IR alters tumor milieu in such a way that TAMΦ aggregate in hypoxic regions and Gr-1+ neutrophils (see Figure 1). 20 Thus, CD68+ TAMΦ, and F4/80+ TAMΦ resegregate into different tumor milieu. It is worth noting that IR-induced hypoxic milieu may have specific factors that cause CD68+ TAMΦ aggregation because avascular chronic hypoxia was observed in larger control tumors, but no CD68+ TAMΦ aggregation. 20
Interestingly, higher Iba-1+ cells were detected at the invading tumor front compared with CD68+, signifying that higher levels of mature MΦ were involved in the invasion of tumor front (see Figure 1). 20 Also, IR recruited the infiltration of more CD68+ MΦ from peripheral blood and these IR-recruited CD68+ MΦ performed the role of M2 MΦ leading to the facilitation of tumor invasion (see Figure 1). 20 Moreover, ALTS1C1 and GL261 tumors were characterized with different CD68+ TAMΦ–hypoxia association patterns, which were related to three monocytes-associated factors such as SDF-1α, VEGF, as well as matrix metalloproteinase-2 (MMP-2; see Figure 1). 20
The IR was capable of inducing SDF-1α production, which facilitated the homing of hematopoietic progenitor cells toward gliomas as well as augmented vessel formation.20,79 Moreover, SDF-1α in the conditioned medium generated by ALTS1C1 tumor not only augmented MΦ migration toward hypoxia, but also lengthen their survival in hypoxic milieu. 79 In addition, SDF-1α generation by tumor cells was capable of triggering the accumulation of TAMΦ in IR-induced hypoxic regions as it silenced ALTS1C1 tumor development in intramuscular or intracranial pre-IR sites. 20
Inhibition of TAMΦ amassment in hypoxia and tumor development delay was further augmented in SDF-1α-suppressed tumors. 20 Thus, SDF-1α facilitated tumor development in an IR milieu and the association of TAMΦ with hypoxia augmented tumor development rate. Moreover, inhibition of SDF-1α secretion in ALTS1C1 tumors by siRNA triggered a reduction microvascular density, TAMΦ density, as well as tumor invasiveness following IR. 80 Therefore, IR-induced SDF-1α secretion was responsible for the IR-induced augmentation in microvascular density, infiltration of MΦ, and vessel vascularization, which subsequently triggered IR-induced tumor invasiveness.20,65
Hypoxia was capable of inducing iNOS secretion, which triggered TAMΦ migration in tumors (see Figure 1). 22 Also, TAMΦ isolated from IR tumors secreted higher levels of iNOS, arginase 1 (Arg I), as well as cyclooxygenase-2 (COX-2; see Figure 1) compared to un-IR tumors. 81 Interestingly, these factors were more effective in facilitating tumor growth. 81 Also, gene expression levels of CD11b, a marker for myeloid cells of the MΦ lineage, was significantly decreased in the hippocampus of IR mice 7 days post-IR. 14 Contrarily, MΦ markers such as CD11b+F4/80+ that co-secretes green fluorescent protein (GFP) as well as red fluorescent protein (RFP) such as CX3CR1+CCR2+ significantly increased 7 days following IR in tumors (see Figure 1). 14
It is worth noting that blood-born monocytes and MΦ predominantly expressed CCR2 rather than resident cells in the brain and spinal cord (see Figure 1). 82 Also, cranial IR was capable of adequately changing the brain’s milieu to allow for the infiltration of peripherally derived, proinflammatory CCR2+ MΦ. 14 Similarly, secretion of tumor necrosis factor alpha (TNF-α) triggered the secretion of CCL2 in astrocytes (see Figure 1). 83 In addition, augmented secretion of CCL2 triggered changes in the integrity of the BBB. 84 Moreover, IR was able to induce anti-inflammatory gene signature in TAMΦ in two spontaneous GBM models. 41
Notably, the underlying mechanism was mainly via cell death in IR M0 and M1 MΦ in GL261 GBM model.34,62 Interestingly, TAMΦ phagocytic activity triggered the removal of apoptotic cells in in vitro human GBM cell lines following IR (see Figure 1). 34 Moreover, a total of 3% of cells were apoptotic cells following IR exposure to the GBM cell lines. 41 Also, higher phagocytic activity triggered the secretion of higher levels of “eat-me” receptors such as the efferocytosis (the process by which apoptotic cells are removed by phagocytic cells) receptor AXL following MΦ exposed to IR-treated GBM cell conditioned media (see Figure 1). 34 Furthermore, the ability of MΦ to prime T cells was inhibited by IR in GBM. 85 Moreover, IL-8 was detected in necrotic areas of the tumor and around MΦ during immunochemistry analysis in about 50% of the patients with GBM following IR (see Figure 1). 77
MΦ Induce Radiosensitization and Radioresistance
Radiosensitization is a physical, chemical, or pharmacological agent that augments the lethal effects of IR when administered in conjunction with IR. It was established that M1 MΦ are more sensitive to IR than M2 MΦ. 62 Notably, temozolomide (TMZ) is an oral alkylating agent used to treat malignant glioma such as GBM and astrocytomas. 34 Also, concurrent administration of IR and TMZ augmented the phagocytic activity of MΦ against four different human GBM cell lines (see Table 1). 34 Similarly, the MΦ modulated the paracrine effect of GBM cells exposed to IR as well as TMZ and this correlated with the percentage of apoptotic GBM cells (see Table 1). 34
Table 1.
Show the influential effects MΦ induce radiosensitization and radioresistance agents and the mechanisms via which they trigger these effects.
| Agent | Influence via MΦ | IR-induce mechanism of action via MΦ | Citation |
|---|---|---|---|
| TMZ | Radiosensitization | IR and TMZ augmented the phagocytic activity of MΦ against four different human GBM cell lines. | Paolicelli et al 31 |
| MΦ modulated the paracrine effect of GBM cells exposed to IR and TMZ via apoptosis | Paolicelli et al 31 | ||
| NO | Radiosensitization | NO and IR enhancing DNA DSBs, blockade of DNA repair and activation of mitotic catastrophe via TAMΦ in GBM | Semple et al, 83 Roberts et al 84 |
| AMD3100 | Radiosensitization | Inhibition of cross-talk between SDF- 1 and CXCR4 by AMD3100 augmented the effectiveness of IR in GBM | Heckler et al 86 |
| PD-1 | Radiosensitization | PD-L1+ circulating monocyte-derived MΦ are the cells that respond primarily to IR sensitivity during GBM therapy | Lomax et al 87 |
| AntiPD-L1 was able to directly stimulate PD-L1+ MΦ to augment production of cytokines and increase phagocytosis in an ERK signaling-dependent fashion following IR | Lomax et al 87 | ||
| SDF-1/ CXCL12 | Radioresistance | An upsurge of SDF-1α at the tumor invasion front after IR was correlated with the recruitment of TAMΦ as well as radioresistance in a murine glioma model. | Takenaka et al 76 |
| SDF-1/CXCL12 triggers glioma invasion by recruiting MΦ or T-regulatory cell to the peritumoral area, via initiation of interaction between endothelial cells or by marshaling hematopoietic stem cells as well as progenitor cells | Tseng et al 85 | ||
| Mesenchymal cells state | Radioresistance | TAMΦ induces cell differentiation in GBM to a mesenchymal state via generated NF-κB following IR | Miyamoto et al, 30 Wunderlich et al 88 |
| VEGF | Radioresistance | TAMΦ subtypes and a higher microvascular density associated with higher levels of VEGF receptor-1 (VEGFR-1) were observed following IR in glioma | Takenaka et al 76 |
Abbreviations: AMD3100, plerixafor; CXCL12, cyclooxygenase-2; CXCR, chemokine receptor; ERK, extracellular signal-regulated kinase; GBM, glioblastoma multiforme; IR, ionizing radiation or irradiation or radiotherapy; MΦ, macrophages; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; NO, nitric oxide; PD, programmed cell death; SDF, stromal cell-derived factor; TAMΦ, tumor-associated macrophages; TMZ, temozolomide; VEGF, vascular endothelial growth factor.
It is worth noting that the positive correlation between MΦ phagocytic activity and the stimulation of apoptosis in GBM cells by IR and TMZ suggests that apoptotic cells are critical in the modulation of phagocytic activity, whereas necrotic as well as secondary necrotic cells does not. 34 Also, IR facilitated a more immunosuppressive milieu via the stimulation of efferocytosis in TAMΦ and an upsurge of tumor cell engulfment by TAMΦ exhibited detrimental effect of the anti-tumoral immune response in GBM. 34 Similarly, NO was able to stimulate radiosensitization in GBM cells under hypoxic conditions by enhancing DNA DSBs, blockade of DNA repair, as well as activation of mitotic catastrophe via TAMΦ (see Table 1).86,87
It is well established that SDF-1/CXCL12 is able to trigger glioma invasion by recruiting MΦ or T-regulatory cell to the peritumoral area, via initiation of interaction between endothelial cells or by marshaling hematopoietic stem cells as well as progenitor cells. 89 Also, plerixafor (AMD3100) is a macrocyclic compound that is able to irreversibly antagonize against the binding of CXCR4 with its ligand SDF-1/CXCL12 (see Table 1).66,89 Furthermore, the blockade of the cross-talk between SDF- 1 with its receptor, CXCR4, by AMD3100 augmented the effectiveness of IR in GBM (see Table 1). 66
Programmed cell death protein 1 (PD-1) blocks immune responses as well as facilitates self-tolerance via the activation of T cells, apoptosis of antigen-specific T cells as well as blockade of apoptosis of T-regulatory cells. Interestingly, PD-L1+ circulating monocyte-derived MΦ are the cells that respond primarily to sensitivity IR during GBM therapy (see Table 1). 90 Notably, after IR, mouse with GBM responded better to anti-PD-L1 therapy, which precisely target infiltrating PD-L1+ MΦ, than to anti-PD-1 immunotherapy. 91 Thus, patients with GBM that fail anti-PD-1 or anti-PD-L1 monotherapy can still respond to anti-PD-L1 combined with high-dose IR of viable tumor cells. Mechanically, antiPD-L1 was able to directly stimulate PD-L1+ MΦ to augment production of cytokines as well as increase phagocytosis in an ERK signaling-dependent fashion following IR (see Table 1). 90
Radioresistance is a process in which the tumor cells or tissues adapt to the IR-induced changes and develop resistance toward the IR. Notably, MΦ inside the tumor mass are associated with multiple phenomena that include IR resistance. Paradoxically, although some studies have described MΦ as a radioresistant cell type,71,88 other studies detected either an upsurge in MΦ in the tumor following x-IR or a reduction. 70 However, MΦ facilitate tumor development as well as represent a negative prognostic factor due to the occurrence of M2 MΦ.56,92 Also, IR triggers changes in the tumor milieu resulting in tumor aggressiveness and recurrence typically occurring near the IR area. 93
It is worth noting that IR triggered a quick inflammatory response resulting in TAMΦ recruitment at tumor milieu and this inflammatory response correlated with a short survival time. 77 Moreover, TAMΦ were capable of inducing cell differentiation in GBM to a mesenchymal state via generated NF-kB, and this correlated with IR resistance (see Table 1). 94 Furthermore, a positive modulation of MΦ chemotaxis, which triggered radioresistance in GBM model was detected via total RNA sequencing following IR exposure. 95
As indicated early, the response of TAMΦ to IR-induced milieu changes in the primary tumor core and the tumor-invading front were different though IR augmented the TAMΦ density in both the primary tumor core as well as the tumor-invading front.20,65 Also, the invading tumor front has a distinct milieu from that of the primary tumor core in ALTS1C1 tumor model because a different ratio of the TAMΦ subtypes and a higher microvascular density associated with higher levels of VEGF receptor-1 (VEGFR-1; see Table 1) and SDF-1 secretion were observed following IR. 79 Moreover, an upsurge of SDF-1α at the tumor invasion front after IR correlated with the recruitment of TAMΦ as well as radioresistance in a murine glioma model. 79
Notably, exposure of IR to the tumor induced modification of multiple pathways and triggered changes in the MΦ activation type, making them more supportive of tumor growth. 96 Also, an increase in M1 MΦ augmentation score correlated with a poor prognosis in GBM patients following IR during a subgroup analysis. 96 Thus, GBM patients with elevated M1 MΦ infiltration had a poorer survival rate. In addition, the subgroup analysis revealed that MΦ were more augmented in IDH-mutant patients, compared to IDH-wild type patients. 97 Thus, GBM patients with IDH mutation had a better survival. Moreover, M1 MΦ had unfavorable prognosis following IR in IDH-wild type GBM patients. 96 Thus, in GBM, M1 MΦ targeted therapies are potential sensitization for IR.
MΦ and IR-Induced Abscopal and Bystander Effects
The abscopal effect occurs when IR shrinks the targeted tumor as well as untreated tumors elsewhere in the body. Thus, any substantial enhancement in survival for GBM patients will necessitate support from the immune system to kill resistant/residual tumor cells outside prior treatment regions. 90 It is worth noting that specific mechanism associated with the abscopal phenomenon remains a paradox. However, IR exposure to tumor triggers a systemic immune response to un-IR and distant tumor foci. 98 Remarkably, the relative influence of antigen-presenting cells (APCs) such as MΦ, dendritic cells, or T cells to the abscopal response differs depending on type of IR, dosage, animal model, as well as immune checkpoint blockers. 99
The IR-induced tumor death triggered the secretion of neo-antigens or neo-epitopes, which were engulfed by APCs prior to T-cell stimulation during the abscopal response. 100 Furthermore, APCs subsequently entered and circulated then to lymph nodes where neo-antigen presentation triggered the stimulation as well as education of naïve T cells. 90 Moreover, stimulated as well as tumor-specific T cells join the circulation and selectively target tumor cells, leading to regression of un-IR tumors. 90 Notably, GBM with neo-epitopes such as epidermal growth factor receptor variant III (EGFRvIII) tumors were more susceptible to immune-related abscopal response 90 and direct stimulation of MΦ was associated with the abscopal response in the absence of T-cell infiltration. 101
The IR-induced bystander effect (IRIBE) is the phenomenon in which non-IR cells show effects along with their different intensities due to signals received from nearby IR cells.70,102 Notably, following WBRT, an IRIBE is often observed in the blood vessels and blood components because the brain is vascularized and contain blood. Interestingly, IRIBE is capable of triggering a sequence of biological endpoints such as augmented micronuclei formation, sister chromatid exchanges, carcinogenesis, as well as decreased cell survival. 102 Thus, IR-induced blood injury is a critical health risk to glioma patient receiving IR.
Specifically, lymphocytes and MΦ are two key constituents in the blood that interrelate with each other as well as influence body organs via blood flow. 70 Notably, MΦ are crucial triggers of bystander signaling factors, and are therefore critical in IRIBE following IR exposure to tissues. 103 Also, lymphocytes are exceedingly sensitive cells that frequently interact with neighboring MΦ in the body following IR. 104 It has been speculated that MΦ are refined by tumor antigens released following IR resulting in a tumor-specific response. 70
Interestingly, infiltrating MΦ are differentiated by anti-PD-L1 antibodies into anti-tumor states resulting in suppression of previously viable tumor cells just outside of the IR area following IR-induced their recruitment in brain. 90 Also, the effect of anti-PD-1 was not similar to anti-PD-L1 following IR exposure to GBM tumor model. 90 Furthermore, MΦ are resistant to inhibition of metabolic activity by IR with low energy carbon ions. However, there were no differences in the consequences of equivalent doses of x-IR or carbon ions on MΦ as measured in the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolimbromid (MTT) test after IR.71,105
It is worth noting that over-secretion of manganese superoxide dismutase (MnSOD) facilitated resistance of cells as well as tissues to IR.105,106 Also, MnSOD–plasmid–liposome complexes triggered substantial protection of cells and tissues from IR damage. 105 Furthermore, over-secretion of the MnSOD transgene triggered an unexpected upsurge in tumor cell toxicity as well as radiosensitization via the generation of H2O2 by the action of MnSOD. 107 Interestingly, MΦ were capable of generating MnSOD in response to IR and this preserved the cells from the harmful effects of IR-induced radical. 108
Also, MnSOD was induced by various agonists such as lipopolysaccharides (LPS), proinflammatory cytokines, hypoxia, and IR. 71 Notably, the generation of MnSOD was triggered by IR resistance. 71 Similarly, IR of RAW 264.7 MΦ alone with either x-IR or carbon ions did not trigger the generation of the proinflammatory cytokines such as TNF-α and IL-1β or the ROS and NO. 71 Moreover, stimulation of murine as well as rat MΦ triggered iNOS generation via several substances such as LPS, IL-1β, IL-6, IFN-γ, TNF-α, or by oxidative stress, whereas human cells require a multifaceted cytokine amalgamation for iNOS stimulation following cranial IR.71,109
Conclusions and Perceptive
Notably, cranial-IR is capable of inducing the migration of peripherally derived MΦ into the brain parenchyma. Moreover, acute response to IR exposure was responsible for the induced peripheral MΦ immigration into the brain. Also, MΦ inside the tumor mass are associated with multiple phenomena that include IR resistance and enrichment of the M2 MΦ after IR is able to facilitate GBM recurrence. In addition, MΦ are crucial triggers of bystander signaling factors and, are therefore critical in IRIBE following IR exposure to tissues. Therefore, future studies ought to focus on the biomarker role of peripheral MΦ following cranial-IR therapy for glioma.
TAMΦ were not brain-resident microglia, but mainly monocyte-derived MΦ from peripheral blood in GBM milieu. IR facilitated a more immunosuppressive milieu via the stimulation of efferocytosis in TAMΦ and an upsurge of tumor cell engulfment by TAMΦ exhibited detrimental effect of the anti-tumoral immune response in GBM. In addition, IR exposure to tumor triggers a systemic immune response to un-IR and distant tumor foci in GBM. Furthermore, IR triggered a quick inflammatory response resulting in TAMΦ recruitment at tumor milieu and this inflammatory response correlated with a short survival time. In GBM, M1 MΦ targeted therapies are potential sensitization for IR. Therefore, future glioma therapeutic agents ought to include agents that are capable of altering the TAMΦ in glioma.
Supplemental Material
Supplemental material, sj-eddx-1-onc-10.1177_11795549241282098 for Pivotal Role of Cranial Irradiation-Induced Peripheral, Intrinsic, and Brain-Engrafting Macrophages in Malignant Glioma by Seidu A Richard, Sagor Kumar Roy and Emmanuel Akomanin Asiamah in Clinical Medicine Insights: Oncology
Acknowledgments
Not applicable.
Footnotes
Author Contributions: Study concepts and design by SAR, SKR, and EAA. Data acquisition by SAR. Article preparation by SAR. Article editing by SAR, SKR, and EAA. All authors carefully reviewed the article and approved the final version and agree to be accountable for all aspects of the work.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Data Availability: No data were used in this article.
Ethics Approval and Consent to Participate: Not applicable.
Consent for Publication: Not applicable.
ORCID iD: Seidu A Richard  https://orcid.org/0000-0003-3475-0363
https://orcid.org/0000-0003-3475-0363
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Mesti T, Ocvirk J. Malignant gliomas: old and new systemic treatment approaches. Radiol Oncol. 2016;50:129-138. doi: 10.1515/raon-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97-109. doi: 10.1007/s00401-007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Louis DN, Perry A, Wesseling P, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 2021;23:1231-1251. doi: 10.1093/neuonc/noab106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Richard SA. EPAC2: a new and promising protein for glioma pathogenesis and therapy. Oncol Rev. 2020;14:446. doi: 10.4081/oncol.2020.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Frosina G. Advancements in image-based models for high-grade gliomas might be accelerated. Cancers. 2024;16:1566. doi: 10.3390/cancers16081566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barnholtz-Sloan JS, Ostrom QT, Cote D. Epidemiology of brain tumors. Neurol Clin. 2018;36:395-419. doi: 10.1016/j.ncl.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 7. Koshy M, Villano JL, Dolecek TA, et al. Improved survival time trends for glioblastoma using the SEER 17 population-based registries. J Neurooncol. 2012;107:207-212. doi: 10.1007/s11060-011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Grochans S, Cybulska AM, Simińska D, et al. Epidemiology of glioblastoma multiforme-literature review. Cancers. 2022;14:2412. doi: 10.3390/cancers14102412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Richard SA. Advances in synthetic lethality modalities for glioblastoma multiforme. Open Med (Wars). 2024;19:20240981. doi: 10.1515/med-2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tran B, Rosenthal MA. Survival comparison between glioblastoma multiforme and other incurable cancers. J Clin Neurosci. 2010;17:417-421. doi: 10.1016/j.jocn.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 11. Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423-1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Richard SA, Sackey M, Kortei NK. Exploring the pivotal neurophysiologic and therapeutic potentials of vitamin C in glioma. J Oncol. 2021;2021:6141591. [Google Scholar]
- 13. Grégoire H, Roncali L, Rousseau A, et al. Targeting tumor associated macrophages to overcome conventional treatment resistance in glioblastoma. Front Pharmacol. 2020;11:368. doi: 10.3389/fphar.2020.00368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Morganti JM, Jopson TD, Liu S, Gupta N, Rosi S. Cranial irradiation alters the brain’s microenvironment and permits CCR2+ macrophage infiltration. PLoS ONE. 2014;9:e93650. doi: 10.1371/journal.pone.0093650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Abayomi OK. Pathogenesis of irradiation-induced cognitive dysfunction. Acta Oncol. 1996;35:659-663. doi: 10.3109/02841869609083995. [DOI] [PubMed] [Google Scholar]
- 16. Hussain SF, Yang D, Suki D, Aldape K, Grimm E, Heimberger AB. The role of human glioma-infiltrating microglia/macrophages in mediating antitumor immune responses. Neuro Oncol. 2006;8:261-279. doi: 10.1215/15228517-2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496:445-455. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mantovani A, Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol. 2010;22:231-237. doi: 10.1016/j.coi.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 19. Richard SA. The pivotal immunoregulatory functions of microglia and macrophages in glioma pathogenesis and therapy. J Oncol. 2022;2022:8903482. doi: 10.1155/2022/8903482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chiang CS, Fu SY, Wang SC, et al. Irradiation promotes an m2 macrophage phenotype in tumor hypoxia. Front Oncol. 2012;2:89. doi: 10.3389/fonc.2012.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549-555. doi: 10.1016/s1471-4906(02)02302. [DOI] [PubMed] [Google Scholar]
- 22. Weigert A, Brüne B. Nitric oxide, apoptosis and macrophage polarization during tumor progression. Nitric Oxide. 2008;19:95-102. doi: 10.1016/j.niox.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 23. Lewis C, Murdoch C. Macrophage responses to hypoxia: implications for tumor progression and anti-cancer therapies. Am J Pathol. 2005;167:627-635. doi: 10.1016/s0002-9440(10)62038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Montemurro N, Pahwa B, Tayal A, et al. Macrophages in recurrent glioblastoma as a prognostic factor in the synergistic system of the tumor microenvironment. Neurol Int. 2023;15:595-608. doi: 10.3390/neurolint15020037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Feng X, Frias ES, Paladini MS, et al. Functional role of brain-engrafted macrophages against brain injuries. J Neuroinflammation. 2021;18:232. doi: 10.1186/s12974-021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Goldmann T, Wieghofer P, Jordão MJ, et al. Origin, fate and dynamics of macrophages at central nervous system interfaces. Nat Immunol. 2016;17:797-805. doi: 10.1038/ni.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Aguzzi A, Barres BA, Bennett ML. Microglia: scapegoat, saboteur, or something else? Science. 2013;339:156-161. doi: 10.1126/science.1227901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lannes N, Eppler E, Etemad S, Yotovski P, Filgueira L. Microglia at center stage: a comprehensive review about the versatile and unique residential macrophages of the central nervous system. Oncotarget. 2017;8:114393-114413. doi: 10.18632/oncotarget.23106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Prionisti I, Bühler LH, Walker PR, Jolivet RB. Harnessing microglia and macrophages for the treatment of glioblastoma. Front Pharmacol. 2019;10:506. doi: 10.3389/fphar.2019.00506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Miyamoto A, Wake H, Ishikawa AW, et al. Microglia contact induces synapse formation in developing somatosensory cortex. Nat Commun. 2016;7:12540. doi: 10.1038/ncomms12540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Paolicelli RC, Bolasco G, Pagani F, et al. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333:1456-1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- 32. Tremblay MÈ. Microglial functional alteration and increased diversity in the challenged brain: insights into novel targets for intervention. Brain Behav Immun Health. 2021;16:100301. doi: 10.1016/j.bbih.2021.100301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhou W, Ke SQ, Huang Z, et al. Periostin secreted by glioblastoma stem cells recruits M2 tumour-associated macrophages and promotes malignant growth. Nat Cell Biol. 2015;17:170-182. doi: 10.1038/ncb3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lecoultre M, Chliate S, Espinoza FI, Tankov S, Dutoit V, Walker PR. Radio-chemotherapy of glioblastoma cells promotes phagocytosis by macrophages in vitro. Radiother Oncol. 2024;190:110049. doi: 10.1016/j.radonc.2023.110049. [DOI] [PubMed] [Google Scholar]
- 35. Silvin A, Qian J, Ginhoux F. Brain macrophage development, diversity and dysregulation in health and disease. Cell Mol Immunol. 2023;20:1277-1289. doi: 10.1038/s41423-023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Woolf Z, Swanson MEV, Smyth LC, et al. Single-cell image analysis reveals a protective role for microglia in glioblastoma. Neurooncol Adv. 2021;3:vdab031. doi: 10.1093/noajnl/vdab031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Landry AP, Balas M, Alli S, Spears J, Zador Z. Distinct regional ontogeny and activation of tumor associated macrophages in human glioblastoma. Sci Rep. 2020;10:19542. doi: 10.1038/s41598-020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gosselin D, Link VM, Romanoski CE, et al. Environment drives selection and function of enhancers controlling tissue-specific macrophage identities. Cell. 2014;159:1327-1340. doi: 10.1016/j.cell.2014.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Buttgereit A, Lelios I, Yu X, et al. Sall1 is a transcriptional regulator defining microglia identity and function. Nat Immunol. 2016;17:1397-1406. doi: 10.1038/ni.3585. [DOI] [PubMed] [Google Scholar]
- 40. Shemer A, Grozovski J, Tay TL, et al. Engrafted parenchymal brain macrophages differ from microglia in transcriptome, chromatin landscape and response to challenge. Nat Commun. 2018;9:5206. doi: 10.1038/s41467-018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Akkari L, Bowman RL, Tessier J, et al. Dynamic changes in glioma macrophage populations after radiotherapy reveal CSF-1R inhibition as a strategy to overcome resistance. Sci Transl Med. 2020;12:eaaw7843. doi: 10.1126/scitranslmed.aaw7843. [DOI] [PubMed] [Google Scholar]
- 42. Chitu V, Gokhan Ş, Nandi S, Mehler MF, Stanley ER. Emerging roles for CSF-1 receptor and its ligands in the nervous system. Trends Neurosci. 2016;39:378-393. doi: 10.1016/j.tins.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cronk JC, Filiano AJ, Louveau A, et al. Peripherally derived macrophages can engraft the brain independent of irradiation and maintain an identity distinct from microglia. J Exp Med. 2018;215:1627-1647. doi: 10.1084/jem.20180247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Varvel NH, Grathwohl SA, Baumann F, et al. Microglial repopulation model reveals a robust homeostatic process for replacing CNS myeloid cells. Proc Natl Acad Sci USA. 2012;109:18150-18155. doi: 10.1073/pnas.1210150109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Priller J, Flügel A, Wehner T, et al. Targeting gene-modified hematopoietic cells to the central nervous system: use of green fluorescent protein uncovers microglial engraftment. Nat Med. 2001;7:1356-1361. doi: 10.1038/nm1201-1356. [DOI] [PubMed] [Google Scholar]
- 46. Kleihues P, Louis DN, Scheithauer BW, et al. The WHO classification of tumors of the nervous system. J Neuropathol Exp Neurol. 2002;61:215-225. [DOI] [PubMed] [Google Scholar]
- 47. Kleihues P, Burger PC, Scheithauer BW. The new WHO classification of brain tumours. Brain Pathol. 1993;3:255-268. [DOI] [PubMed] [Google Scholar]
- 48. Gladson CL, Prayson RA, Liu WM. The pathobiology of glioma tumors. Annu Rev Pathol. 2010;5:33-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131:803-820. doi: 10.1007/s00401-016. [DOI] [PubMed] [Google Scholar]
- 50. Wesseling P, Capper D. WHO 2016 classification of gliomas. Neuropathol Appl Neurobiol. 2018;44:139-150. doi: 10.1111/nan.12432. [DOI] [PubMed] [Google Scholar]
- 51. Ohgaki H, Kleihues P. The definition of primary and secondary glioblastoma. Clin Cancer Res. 2013;19:764-772. doi: 10.1158/1078-0432.Ccr. [DOI] [PubMed] [Google Scholar]
- 52. Müller S, Kohanbash G, Liu SJ, et al. Single-cell profiling of human gliomas reveals macrophage ontogeny as a basis for regional differences in macrophage activation in the tumor microenvironment. Genome Biol. 2017;18:234. doi: 10.1186/s13059-017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pombo Antunes AR, Scheyltjens I, Lodi F, et al. Single-cell profiling of myeloid cells in glioblastoma across species and disease stage reveals macrophage competition and specialization. Nat Neurosci. 2021;24:595-610. doi: 10.1038/s41593-020. [DOI] [PubMed] [Google Scholar]
- 54. Neftel C, Laffy J, Filbin MG, et al. An integrative model of cellular states, plasticity, and genetics for glioblastoma. Cell. 2019;178:835-849.e821. doi: 10.1016/j.cell.2019.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kimura YN, Watari K, Fotovati A, et al. Inflammatory stimuli from macrophages and cancer cells synergistically promote tumor growth and angiogenesis. Cancer Sci. 2007;98:2009-2018. doi: 10.1111/j.1349-7006.2007.00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Prosniak M, Harshyne LA, Andrews DW, et al. Glioma grade is associated with the accumulation and activity of cells bearing M2 monocyte markers. Clin Cancer Res. 2013;19:3776-3786. doi: 10.1158/1078-0432.Ccr. [DOI] [PubMed] [Google Scholar]
- 57. Chen R, Smith-Cohn M, Cohen AL, Colman H. Glioma subclassifications and their clinical significance. Neurotherapeutics. 2017;14:284-297. doi: 10.1007/s13311-017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ochocka N, Segit P, Walentynowicz KA, et al. Single-cell RNA sequencing reveals functional heterogeneity of glioma-associated brain macrophages. Nat Commun. 2021;12:1151. doi: 10.1038/s41467-021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Engler JR, Robinson AE, Smirnov I, et al. Increased microglia/macrophage gene expression in a subset of adult and pediatric astrocytomas. PLoS ONE. 2012;7:e43339. doi: 10.1371/journal.pone.0043339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zeiner PS, Preusse C, Golebiewska A, et al. Distribution and prognostic impact of microglia/macrophage subpopulations in gliomas. Brain Pathol. 2019;29:513-529. doi: 10.1111/bpa.12690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Nishie A, Ono M, Shono T, et al. Macrophage infiltration and heme oxygenase-1 expression correlate with angiogenesis in human gliomas. Clin Cancer Res. 1999;5:1107-1113. [PubMed] [Google Scholar]
- 62. Leblond MM, Pérès EA, Helaine C, et al. M2 macrophages are more resistant than M1 macrophages following radiation therapy in the context of glioblastoma. Oncotarget. 2017;8:72597-72612. doi: 10.18632/oncotarget.19994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Scott CL, Zheng F, De Baetselier P, et al. Bone marrow-derived monocytes give rise to self-renewing and fully differentiated Kupffer cells. Nat Commun. 2016;7:10321. doi: 10.1038/ncomms10321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ohsawa K, Imai Y, Sasaki Y, Kohsaka S. Microglia/macrophage-specific protein Iba1 binds to fimbrin and enhances its actin-bundling activity. J Neurochem. 2004;88:844-856. doi: 10.1046/j.1471-4159.2003.02213.x. [DOI] [PubMed] [Google Scholar]
- 65. Wang SC, Yu CF, Hong JH, Tsai CS, Chiang CS. Radiation therapy-induced tumor invasiveness is associated with SDF-1-regulated macrophage mobilization and vasculogenesis. PLoS ONE. 2013;8:e69182. doi: 10.1371/journal.pone.0069182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kioi M, Vogel H, Schultz G, Hoffman RM, Harsh GR, Brown JM. Inhibition of vasculogenesis, but not angiogenesis, prevents the recurrence of glioblastoma after irradiation in mice. J Clin Invest. 2010;120:694-705. doi: 10.1172/jci40283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Chiang CS, Hong JH, Stalder A, Sun JR, Withers HR, McBride WH. Delayed molecular responses to brain irradiation. Int J Radiat Biol. 1997;72:45-53. doi: 10.1080/095530097143527. [DOI] [PubMed] [Google Scholar]
- 68. Leblond MM, Gérault AN, Corroyer-Dulmont A, et al. Hypoxia induces macrophage polarization and re-education toward an M2 phenotype in U87 and U251 glioblastoma models. Oncoimmunology. 2016;5:e1056442. doi: 10.1080/2162402x.2015.1056442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Narita T, Aoyama H, Hirata K, et al. Reoxygenation of glioblastoma multiforme treated with fractionated radiotherapy concomitant with temozolomide: changes defined by 18F-fluoromisonidazole positron emission tomography: two case reports. Jpn J Clin Oncol. 2012;42:120-123. doi: 10.1093/jjco/hyr181. [DOI] [PubMed] [Google Scholar]
- 70. Dong C, He M, Ren R, et al. Role of the MAPK pathway in the observed bystander effect in lymphocytes co-cultured with macrophages irradiated with γ-rays or carbon ions. Life Sci. 2015;127:19-25. doi: 10.1016/j.lfs.2015.02.017. [DOI] [PubMed] [Google Scholar]
- 71. Conrad S, Ritter S, Fournier C, Nixdorff K. Differential effects of irradiation with carbon ions and x-rays on macrophage function. J Radiat Res. 2009;50:223-231. doi: 10.1269/jrr.08115. [DOI] [PubMed] [Google Scholar]
- 72. Prakash H, Klug F, Nadella V, Mazumdar V, Schmitz-Winnenthal H, Umansky L. Low doses of gamma irradiation potentially modifies immunosuppressive tumor microenvironment by retuning tumor-associated macrophages: lesson from insulinoma. Carcinogenesis. 2016;37:301-313. doi: 10.1093/carcin/bgw007. [DOI] [PubMed] [Google Scholar]
- 73. Wang X, Ning W, Qiu Z, Li S, Zhang H, Yu C. Tumor-associated macrophages based signaling pathway analysis and hub genes identification in glioma. Medicine (Baltimore). 2020;99:e23840. doi: 10.1097/md.0000000000023840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hambardzumyan D, Gutmann DH, Kettenmann H. The role of microglia and macrophages in glioma maintenance and progression. Nat Neurosci. 2016;19:20-27. doi: 10.1038/nn.4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wang J, Mi S, Ding M, Li X, Yuan S. Metabolism and polarization regulation of macrophages in the tumor microenvironment. Cancer Lett. 2022;543:215766. doi: 10.1016/j.canlet.2022.215766. [DOI] [PubMed] [Google Scholar]
- 76. Takenaka MC, Gabriely G, Rothhammer V, et al. Control of tumor-associated macrophages and T cells in glioblastoma via AHR and CD39. Nat Neurosci. 2019;22:729-740. doi: 10.1038/s41593-019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Tabatabaei P, Visse E, Bergström P, Brännström T, Siesjö P, Bergenheim AT. Radiotherapy induces an immediate inflammatory reaction in malignant glioma: a clinical microdialysis study. J Neurooncol. 2017;131:83-92. doi: 10.1007/s11060-016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zagzag D, Lukyanov Y, Lan L, et al. Hypoxia-inducible factor 1 and VEGF upregulate CXCR4 in glioblastoma: implications for angiogenesis and glioma cell invasion. Lab Invest. 2006;86:1221-1232. doi: 10.1038/labinvest.3700482. [DOI] [PubMed] [Google Scholar]
- 79. Wang SC, Hong JH, Hsueh C, Chiang CS. Tumor-secreted SDF-1 promotes glioma invasiveness and TAM tropism toward hypoxia in a murine astrocytoma model. Lab Invest. 2012;92:151-162. doi: 10.1038/labinvest.2011.128. [DOI] [PubMed] [Google Scholar]
- 80. Hoelzinger DB, Demuth T, Berens ME. Autocrine factors that sustain glioma invasion and paracrine biology in the brain microenvironment. J Natl Cancer Inst. 2007;99:1583-1593. doi: 10.1093/jnci/djm187. [DOI] [PubMed] [Google Scholar]
- 81. Tsai CS, Chen FH, Wang CC, et al. Macrophages from irradiated tumors express higher levels of iNOS, arginase-I and COX-2, and promote tumor growth. Int J Radiat Oncol Biol Phys. 2007;68:499-507. doi: 10.1016/j.ijrobp.2007.01.041. [DOI] [PubMed] [Google Scholar]
- 82. Varol C, Yona S, Jung S. Origins and tissue-context-dependent fates of blood monocytes. Immunol Cell Biol. 2009;87:30-38. doi: 10.1038/icb.2008.90. [DOI] [PubMed] [Google Scholar]
- 83. Semple BD, Frugier T, Morganti-Kossmann MC. CCL2 modulates cytokine production in cultured mouse astrocytes. J Neuroinflammation. 2010;7:67. doi: 10.1186/1742-2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Roberts TK, Eugenin EA, Lopez L, et al. CCL2 disrupts the adherens junction: implications for neuroinflammation. Lab Invest. 2012;92:1213-1233. doi: 10.1038/labinvest.2012.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Tseng D, Volkmer JP, Willingham SB, et al. Anti-CD47 antibody-mediated phagocytosis of cancer by macrophages primes an effective antitumor T-cell response. Proc Natl Acad Sci USA. 2013;110:11103-11108. doi: 10.1073/pnas.1305569110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Heckler M, Osterberg N, Guenzle J, et al. The nitric oxide donor JS-K sensitizes U87 glioma cells to repetitive irradiation. Tumour Biol. 2017;39:1010428317703922. doi: 10.1177/1010428317703922. [DOI] [PubMed] [Google Scholar]
- 87. Lomax ME, Folkes LK, O’Neill P. Biological consequences of radiation-induced DNA damage: relevance to radiotherapy. Clin Oncol (R Coll Radiol). 2013;25:578-585. doi: 10.1016/j.clon.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 88. Wunderlich R, Ernst A, Rödel F, et al. Low and moderate doses of ionizing radiation up to 2 Gy modulate transmigration and chemotaxis of activated macrophages, provoke an anti-inflammatory cytokine milieu, but do not impact upon viability and phagocytic function. Clin Exp Immunol. 2015;179:50-61. doi: 10.1111/cei.12344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Pusic I, DiPersio JF. Update on clinical experience with AMD3100, an SDF-1/CXCL12-CXCR4 inhibitor, in mobilization of hematopoietic stem and progenitor cells. Curr Opin Hematol. 2010;17:319-326. doi: 10.1097/MOH.0b013e328338b7d5. [DOI] [PubMed] [Google Scholar]
- 90. Ene CI, Kreuser SA, Jung M, et al. Anti-PD-L1 antibody direct activation of macrophages contributes to a radiation-induced abscopal response in glioblastoma. Neuro Oncol. 2020;22:639-651. doi: 10.1093/neuonc/noz226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Burrell K, Hill RP, Zadeh G. High-resolution in-vivo analysis of normal brain response to cranial irradiation. PLoS ONE. 2012;7:e38366. doi: 10.1371/journal.pone.0038366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Lu-Emerson C, Snuderl M, Kirkpatrick ND, et al. Increase in tumor-associated macrophages after antiangiogenic therapy is associated with poor survival among patients with recurrent glioblastoma. Neuro Oncol. 2013;15:1079-1087. doi: 10.1093/neuonc/not082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Gupta K, Burns TC. Radiation-induced alterations in the recurrent glioblastoma microenvironment: therapeutic implications. Front Oncol. 2018;8:503. doi: 10.3389/fonc.2018.00503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Bhat KPL, Balasubramaniyan V, Vaillant B, et al. Mesenchymal differentiation mediated by NF-κB promotes radiation resistance in glioblastoma. Cancer Cell. 2013;24:331-346. doi: 10.1016/j.ccr.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Doan NB, Nguyen HS, Alhajala HS, et al. Identification of radiation responsive genes and transcriptome profiling via complete RNA sequencing in a stable radioresistant U87 glioblastoma model. Oncotarget. 2018;9:23532-23542. doi: 10.18632/oncotarget.25247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Zhou Z, Wen L, Lai M, et al. Increased M1 macrophages infiltration correlated with poor survival outcomes and radiation response in gliomas. Dose Response. 2020;18:1559325820964991. doi: 10.1177/1559325820964991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Venteicher AS, Tirosh I, Hebert C, et al. Decoupling genetics, lineages, and microenvironment in IDH-mutant gliomas by single-cell RNA-seq. Science. 2017;355:eaai8478. doi: 10.1126/science.aai8478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Mole RH. Whole body irradiation; radiobiology or medicine? Br J Radiol. 1953;26:234-241. doi: 10.1259/0007-1285. [DOI] [PubMed] [Google Scholar]
- 99. Marconi R, Strolin S, Bossi G, Strigari L. A meta-analysis of the abscopal effect in preclinical models: is the biologically effective dose a relevant physical trigger. PLoS ONE. 2017;12:e0171559. doi: 10.1371/journal.pone.0171559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Ngwa W, Irabor OC, Schoenfeld JD, Hesser J, Demaria S, Formenti SC. Using immunotherapy to boost the abscopal effect. Nat Rev Cancer. 2018;18:313-322. doi: 10.1038/nrc.2018.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Huang Y, Lee C, Borgström P, Gjerset RA. Macrophage-mediated bystander effect triggered by tumor cell apoptosis. Mol Ther. 2007;15:524-533. doi: 10.1038/sj.mt.6300080. [DOI] [PubMed] [Google Scholar]
- 102. Mancuso M, Pasquali E, Leonardi S, et al. Oncogenic bystander radiation effects in Patched heterozygous mouse cerebellum. Proc Natl Acad Sci USA. 2008;105:12445-12450. doi: 10.1073/pnas.0804186105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Coates PJ, Rundle JK, Lorimore SA, Wright EG. Indirect macrophage responses to ionizing radiation: implications for genotype-dependent bystander signaling. Cancer Res. 2008;68:450-456. doi: 10.1158/0008-5472.Can-07-3050. [DOI] [PubMed] [Google Scholar]
- 104. Degan P, Sancandi M, Zunino A, et al. Exposure of human lymphocytes and lymphoblastoid cells to simulated microgravity strongly affects energy metabolism and DNA repair. J Cell Biochem. 2005;94:460-469. doi: 10.1002/jcb.20302. [DOI] [PubMed] [Google Scholar]
- 105. Epperly MW, Bernarding M, Gretton J, Jefferson M, Nie S, Greenberger JS. Overexpression of the transgene for manganese superoxide dismutase (MnSOD) in 32D cl 3 cells prevents apoptosis induction by TNF-alpha, IL-3 withdrawal, and ionizing radiation. Exp Hematol. 2003;31:465-474. doi: 10.1016/s0301-472x(03)00041. [DOI] [PubMed] [Google Scholar]
- 106. Wong GH. Protective roles of cytokines against radiation: induction of mitochondrial MnSOD. Biochim Biophys Acta. 1995;1271:205-209. doi: 10.1016/0925-4439(95)00029. [DOI] [PubMed] [Google Scholar]
- 107. Epperly MW, Wegner R, Kanai AJ, et al. Effects of MnSOD-plasmid liposome gene therapy on antioxidant levels in irradiated murine oral cavity orthotopic tumors. Radiat Res. 2007;167:289-297. doi: 10.1667/rr0761.1. [DOI] [PubMed] [Google Scholar]
- 108. Hachiya M, Shimizu S, Osawa Y, Akashi M. Endogenous production of tumour necrosis factor is required for manganese superoxide dismutase expression by irradiation in the human monocytic cell line THP-1. Biochem J. 1997;328:615-623. doi: 10.1042/bj3280615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. De Ridder M, Verovski VN, Darville MI, et al. Macrophages enhance the radiosensitizing activity of lipid A: a novel role for immune cells in tumor cell radioresponse. Int J Radiat Oncol Biol Phys. 2004;60:598-606. doi: 10.1016/j.ijrobp.2004.05.065. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-eddx-1-onc-10.1177_11795549241282098 for Pivotal Role of Cranial Irradiation-Induced Peripheral, Intrinsic, and Brain-Engrafting Macrophages in Malignant Glioma by Seidu A Richard, Sagor Kumar Roy and Emmanuel Akomanin Asiamah in Clinical Medicine Insights: Oncology