Abstract
Background
This case-control study investigated the relationship between sleep duration and quality with the occurrence of breast cancer among women, both pre- and post-menopausal, in the northwest Khyber Pakhtunkhwa (KP) region of Pakistan.
Method
This case-control research was carried in multiple tertiary care facilities. Newly diagnosed primary breast cancer patients were recruited as cases (n = 408), and 5+ years age-matched controls (n = 408) were randomly selected from the general population. Participants completed a Pittsburg sleeping quality index (PSQI) questionnaire that included questions on sleep characteristics. Statistical analysis included independent t-tests to compare mean sleep durations and quality scores between groups, and logistic regression to adjust for potential confounders.
Results
Sleep onset latency between cases and controls was not significantly associated with health outcomes, with a P-value of .142. However, sleep duration showed a significant association (P = .049). For sleep duration, the adjusted odds ratio for ≤6 h was 1.02 (95% CI: .5-2.1), while for 7-8 h the adjusted odds ratio was 1.0 (95% CI: .6-1.6). Self-reported sleep quality did not demonstrate significant associations, with the P-value for “very good” sleep quality being .561. Sleep duration of less than 6 h among women with triple-negative breast cancer (TNBC) was found to be strongly associated with a more aggressive type of breast cancer, with an adjusted odds ratio of 1.5 (95% CI: 1.02-2.3, P < .05).
Conclusion
This study does not provide evidence to support an association between sleep duration or quality and the risk of breast cancer. However, it reports a significant association, with shorter sleep durations linked to an increased risk particularly in the context of aggressive breast cancer types such as TNBC.
Keywords: breast cancer, case-control study, sleep duration, sleep quality
Introduction
Breast cancer is a clinically and etiologically diverse disease, with risk factors contributing to the development of different tumour subtypes in different ways. 1 Cancer is expected to rise by 70% in emerging countries during the next 20-25 years. 2 Breast cancer accounts for 20-30% of all cancers in women, and is the leading cause of cancer death in women worldwide. 3 Because transitioning from a traditional to a Westernized lifestyle exposes women to higher individual risk, lifestyle changes are likely to play a role. 2 Some studies have revealed a 13-36 min reduction in habitual sleep length over the last 20-30 years (3-7), while sleep duration reductions have not been consistently found. 4 Sleep quality studies have revealed increases in the prevalence of subjective poor-quality sleep ranging from 5% to nearly 40%. 5 There is some evidence linking poor sleep to a variety of long-term health consequences, including an increased risk of cancer (12-18). Impaired immune function and metabolic pathways that contribute to obesity are 2 plausible biological theories that have been offered to explain how inadequate sleep can directly influence the development of cancer. 6 Another indirect mechanism has been hypothesised, which shows that abnormal melatonin release increases the risk of cancer. 7 All 3 suggested pathways have some evidence to back them up, but the exact mechanism through which sleep affects cancer risk is uncertain. 7 The light/dark cycle regulates melatonin release rather than sleep, and past research has utilised sleep as a surrogate for “exposure to darkness.” 8
Melatonin has been proposed as a factor in the link between sleep length and breast cancer. 9 The pineal gland in the brain produces and secretes melatonin (5-methoxytryptamine), which regulates the body’s circadian rhythm. 8 Melatonin may inhibit breast tumorigenesis directly by inhibiting mammary cell proliferation and invasiveness, as well as indirectly by lowering oestrogen levels via a down-regulation of the hypothalamic pituitary reproductive axis and regulating the activity of the aromatases, the enzymes responsible for local oestrogen synthesis. 9 We hypothesize that shorter sleep duration and poor sleep quality are associated with an increased risk of breast cancer. The objective of this research was to look into the link between breast cancer and 2 domains of sleep (sleep duration and subjective sleep quality), in a population of Pakistani women from the northwest Khyber Pakhtunkhwa (KP) region of Pakistan.
Materials and Methods
Study Participants
This was a case-control study conducted at multiple tertiary care facilities in the North West region of KP, Pakistan. STROBE guidelines were followed for this study. 10 Ethical approval was obtained from the Faculty of Nutrition Sciences, Ethics Committee and Human Studies Review Board (FNS-ECHSRB) at The University of Agriculture, Peshawar, Pakistan (approval number No. 2020-02). After obtaining ethical approval, research was conducted at the Oncology Units’ Out Patients Department (OPD) of Institute of radiation and nuclear Medicine (IRNUM), and Khyber Teaching Hospital (KTH), Peshawar. Newly diagnosed cases (within the previous month) women were recruited consecutively based on gender, oncologist-confirmed histology, and the existence of primary breast cancer. Controls were healthy women from the same demographic region, selected during routine medical checkups at general hospitals. The control group was age-matched within ±5 years and from the same area. Despite efforts to match both groups closely in terms of key sociodemographic characteristics, some differences were observed and analyzed in the study. The sample size for the case and control groups was calculated based on Lwanga et al 11 recommendations, aiming for an adjusted odds ratio with 95% confidence and 5% significance, resulting in a total of 816 participants (408 cases and 408 controls). Data were collected using a convenience sampling technique. Informed consent was obtained from all participants.
Socio Economic Status
To collect data on different factors like socio-economics status, education and occupation status, and reproductive characteristics, a standardized questionnaire was created based on these parameters.
Anthropometric Measurements
Using standardized instruments, women’s anthropometric measurements such as weight, height, waist, and hip circumferences were taken. 12 Weight and height measurements were used to compute BMI, while women’s waist and hip measurements were used to determine waist to hip ratio. 13
Sleep Quality Questionnaire
The Pittsburgh Sleep Quality Index was used to assess sleep quality over the course of a month (PSQI). Subjective sleep quality, sleep latency, sleep length, habitual sleep efficiency, sleep disturbances, use of sleep medicine, and excessive daytime drowsiness are among the 19 items on this self-reported validated and widely used questionnaire that make up the 7 component scores. Furthermore, overall self-reported sleep quality was assessed by aggregating component values and sleep duration was defined as sleeping hour 6, between 7 and 8 h, and >8 h.
Statistical Analysis
Data were analyzed using SPSS version 20. Student’s t-tests were used to compare means, while chi-square tests assessed the frequency differences between groups. Logistic regression was used to calculate adjusted odds ratios and confidence intervals.” A P-value of <.05 was considered statistically significant.
Result
Descriptive characteristics of the population by case/control status:
There were 408 of breast cancer confirmed and healthy subjects as shown in Table 1. The education status of the participants was categorized into 2 levels: illiterate and educated. Among BC (Breast Cancer) cases, 59% had no schooling, and 36% had secondary school, intermediate, or higher education. Compared to healthy women, 41% had no schooling, and 64% of the healthy controls had secondary school, intermediate, or higher education (adjusted OR = 2.8, 95% CI: 2.0-3.9, P < .05). Overall, the gap in education level shows that the risk of BC is 2.8 times higher in illiterate women. According to the study’s findings, housewives comprised 53% of BC patients, while employed women made up 33%. Among healthy individuals, housewives accounted for 47%, and employed women for 67% (adjusted OR = 1.8, 95% CI: 1.2-2.9, P < .05). This indicates a significant disparity in occupational status, with housewives being 1.8 times more prone to developing BC compared to working women. The average income in KP is Rs 20,000. The income level between the case and control groups did not show significant results (adjusted OR = 1.0, 95% CI: .7-1.3). Table 1 also depicts the reproductive characteristics of the study participants. The age at first menarche (<12 years) showed a protective effect against BC (adjusted OR = 0.6, 95% CI: .4-.8, P < .05). The age at first pregnancy (>20 years) indicated a 1.0 times higher risk of BC compared to controls (adjusted OR = 1.0, 95% CI: 1.0-1.5, P < .05).
Table 1.
General Characteristics by Case-Control Status (n = 816).
| Characteristics | Mean ± SD/N (%) | |||||
|---|---|---|---|---|---|---|
| Total (N = 816) | Cases (n = 408) | Controls (n = 408) | P-Value | Adjusted** | ||
| Age | 816 | 45 + 6.3 | 45 + 3.3 | .123 | — | |
| Marital status | Single | 86 | 38 (44%) | 48 (56%) | .542 | Reference |
| Married | 730 | 370 (51%) | 36 (49%) | .9 (.6-1.5) | ||
| Education level | Illiterate | 505 | 297 (59%) | 208 (41%) | .001 | 2.8 (2.0-3.9)* |
| Educated | 311 | 111 (36%) | 200 (64%) | Reference | ||
| Occupation status | Housewives | 702 | 370 (53%) | 332 (47%) | .001 | 1.8 (1.2-2.9)* |
| Working | 114 | 38 (33%) | 76 (67%) | Reference | ||
| Income categories | Above/equal 2 | 439 | 206 (47%) | 233 (53%) | .06 | Reference |
| Below | 377 | 202 (54%) | 175 (46%) | 1.0 (.7-1.3) | ||
| Age at menarche (year) | <12 years | 308 | 140 (47%) | 168 (53%) | .035 | Reference |
| >12 years | 508 | 268 (53%) | 240 (47%) | .6 (.4-.8)* | ||
| Age of 1st pregnancy (year) | <20 years | 431 | 199 (46%) | 232 (54%) | .032 | Reference |
| >20 years | 276 | 154 (54%) | 122 (46%) | 1.0 (1.0-1.57)* | ||
| Breastfeeding practices | No/rare | 82 | 49 (14%) | 33 (9%) | .052 | Reference |
| Yes | 628 | 303 (86%) | 325 (91%) | .6 (.4-.9)* | ||
| Menopausal status | Premenopausal <45 year | 390 | 190 (49%) | 200 (51% | .023 | Reference |
| Postmenopausal >45 years | 426 | 218 (51%) | 208 (49%) | 2.9 (1.5-3.9)* | ||
| Family history of cancer | No | 585 | 281 (69%) | 304 (74%) | .631 | Reference |
| Breast cancer | 102 | 62 (16%) | 40 (10%) | 1.8 (.7-2.6) | ||
| Any other cancer | 125 | 61 (15%) | 64 ((16%) | --- | ||
| BMI | Normal | 340 | 97 (24%) | 138 (33%) | .431 | Reference |
| Obese/overweight | 476 | 106 (26%) | 78 (19%) | 1.01 (1-1.04* | ||
| WHR | Normal (<.85) | 431 | 278 (68%) | 304 (74%) | Reference | |
| Higher WHR (>.85) | 385 | 130 (32%) | 104 (26%) | .223 | 2.2 (1.2-3.9)* | |
SD: standard deviation; N%: number of women (percentage), * At α = .05, to compare the 2 groups, student t tests and chi square tests were use2 Average family size of KP ie, 7,2 Average income of KP (2010-11) ie, Rs. 20,130/.
**Adjusted with marital status as single, educated women, working women, low income, **Adjusted with age at first menarche, age at first pregnancy, family history of BC, menopause status and breast-feeding practice.
Among breast cancer women, 86% practiced breastfeeding, while 14% did not (adjusted OR = .6, 95% CI: .4-.9, P < .05). Breastfeeding showed a significant protective effect against BC. Late menopause (>45 years) was significantly associated with BC risk (adjusted OR = 2.9, 95% CI: 1.5-3.9), showing a 2.9 times higher risk compared to menopause before 45 years. As shown in the Table 1, family history of BC had a marginally non-significant result (adjusted OR = 1.8, 95% CI: .7-2.6, P = .06), suggesting it may not be a leading factor in developing BC. Regarding anthropometric status, overweight and obesity were more common among BC cases compared to healthy controls (adjusted OR = 1.01, 95% CI: 1.0-1.04*). Based on WHR classification, there were significantly more women with a higher WHR value among BC cases (OR = 2.2, 95% CI: 1.2-3.9)*.
Sleep Characteristics of the Participants
The results of sleep characteristics shown in Table 2 indicate only significant differences in sleep duration between BC cases and controls, in which BC cases reported significantly less sleep duration as compared to controls (6 h vs 7 h per day, P < .05). There was no statistical difference in the sleep onset latency. Data regarding overall self-reported sleep quality show that sleep quality did not differ between cases and controls (P > .05).
Table 2.
Associations Between Sleep Duration and Subjective Sleep Quality With Risk of BC in Studied Subjects (n = 816).
| Characteristics | Total (N = 816) | Cases (n = 408) | Controls (n = 408) | P-Values* | OR (95% CI) | ||
|---|---|---|---|---|---|---|---|
| Sleep characteristics | Unadjusted | Adjusted** | |||||
| Sleep onset latency (minutes) | 815 | 40 ± 0.32 | 40 ± 0.24 | .142 | — | — | |
| Duration of sleep (hours) | 815 | 6 ± 0.07 | 7 ± 0.06 | .049* | — | — | |
| Sleeping hours | |||||||
| Sleeping hours | ≤6 h | 378 | 191 (47%) | 187 (46%) | .612 | 1.5 (.0-2.3) | 1.02 (.5-2.1) |
| 7 – 8 h | 336 | 158 (39%) | 178 (44%) | 1.3 (.7-1.8) | 1 (.6-1.6) | ||
| >8 h | 98 | 56 (14%) | 42 (10%) | Reference | Reference | ||
| Overall self-reported sleep quality | Very good | 135 | 76 (19%) | 59 (15%) | .561 | Reference | Reference |
| Good | 284 | 130 (32%) | 154 (38%) | .8 (.7-1) | .9 (.7-1.2) | ||
| Bad | 233 | 114 (28%) | 119 (29%) | .9 (.8-1.2) | 1 ( .8-1.3) | ||
| Very bad | 161 | 74 (18%) | 87 (21%) | .8 (.7-1) | .9 (.7-1.2) | ||
*At α = .05, Student t tests or chi-square tests were used to compare group differences, ** adjusted with, age at fist menarche, age at first pregnancy, menopause status, WHR, family history of BC, breast-feeding practices stress level (high), sleep duration (>8 h).
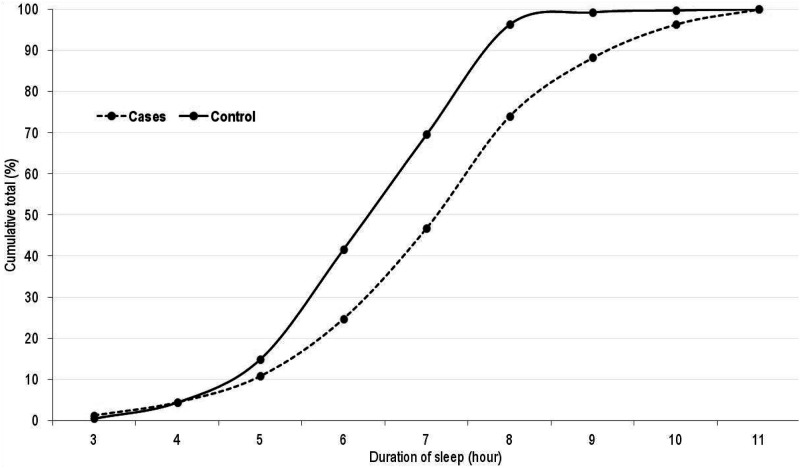
In this population, there is an inverse relationship between the duration of sleep and the incidence of breast cancer, as seen in Figure 1. Sleep length (hours) differed considerably between cases and controls, although most sleep parameters were not found to be significantly associated with breast cancer risk in this population. To learn more about the role of sleep deprivation in breast cancer etiology, more accurate sleep quality assessments are needed.
Figure 1.
Cumulative distribution of sleep duration (hour) by case-control status.
Distribution of Sleep Duration (Hour) by Case-Control Status
Results regarding Immuno histochemical profile or distribution of BC patient’s profiles are presented in Table 3. In this research, breast cancer was classified into 3 types: luminal, HER-2-overexpressing, and basal-like BC. 55-% of patients had Luminal A, and 6-% & 11-% patient from subgroups of Luminal B respectively, 18-% had Triple Negative or Basal-like BC, and 10-% were classified as HER2 Overexpressing.
Table 3.
Clinical Characteristics of Breast Cancer Patients (n = 408).
| Characteristics | N (%) | ||
|---|---|---|---|
| Laterality | Right sided | 170 (42%) | |
| Left sided | 230 (56%) | ||
| Bilateral | 8 (2%) | ||
| Histological grades | Grade – I (low) | 78 (19%) | |
| Grade – II (intermediate) | 262 (65%) | ||
| Grade – III (high) | 63 (16%) | ||
| Molecular subtypes* | Luminal A | 223 (55%) | |
| Luminal B | HER2-positive luminal B (triple positive) | 25 (6%) | |
| HER2-negative luminal B (HR+/HER2−) | 45 (11%) | ||
| Triple negative or basal like | 74 (18%) | ||
| HER2 overexpression | 41 (10%) | ||
HER2: human epidermal growth factor; HR: hormone receptor.
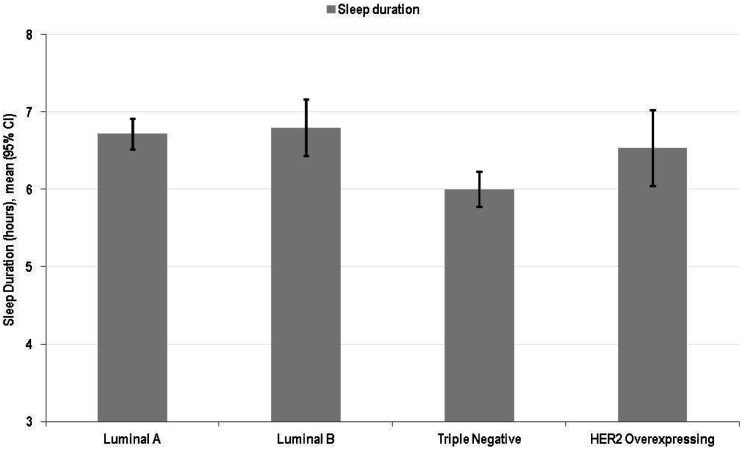
Figure 2 indicates the correlation between molecular subtypes and sleep patterns, with women with triple-negative tumors reporting the highest sleep disruption
Figure 2.
Sleep duration by breast cancer types.
Sleep Duration by Breast Cancer Types
Discussion
In women from Pakistan’s North West of KP region, this research found no significant relationship between sleep duration and the risk of breast cancer, on further sleep quality parameter categorized showed that with participants sleeping 6 h or more everyday having a considerably might elevated risk of breast cancer. An investigation of varied risks for breast cancer found a link between short sleep duration and risk of triple negative breast cancer. These findings are in line with a number of other investigations. In general, (Qian et al, 14 ) reported an inverse relationship between sleep period and the risk of breast cancer in Japanese women, with participants sleeping 6 h or less every day exhibiting a slightly elevated risk of breast cancer development. The current study results are in line with (Jacob et al, 15 ), who found no connection between sleep and risk of BC development, using self-reported sleep quality on a 4-point scale (poor, reasonably poor, reasonably excellent, and sound). An epidemiological study, by (Zaki et al, 16 ) reported sleep characteristics, including usual sleep duration, were not linked to an elevated chance of developing breast cancer. However, not having enough sleep duration and sleep quality was linked to an increased risk of breast cancer. These sleep features may be signs of poor sleep quality. The findings from a study conducted by (Medic et al, 2017) suggest that most sleep characteristics were not found to be closely linked to breast cancer risk. 17 It is clear from the research conducted so far that better measurements for sleep quality are required to get a deeper understanding of the impact of sleep disruption on the etiology of breast cancer. In addition, this study’s findings were in line with those of Vaughn et al, 18 who suggested that sleep problems may be linked to the development of more aggressive TNBC basal-like breast cancers. In addition to pathways involving hormones, short sleep duration has pro-inflammatory and immune-modulatory effects, possibly leading to the development of more aggressive breast tumors. 19 Circadian rhythm is involved in DNA repair, as proteins associated with the biological clock are associated with DNA damage checkpoints 18 Defective pathways lead to increased cancer progression, genetic instability, and abnormalities in chromosomes. 20 These cancer-specific mechanisms support our findings that short sleep duration and poor sleep quality are associated with later stage and triple negative tumors.
Sleep quality is critical for one’s physical and mental health, and sleep deprivation has indeed been attributed to a wide range of chronic diseases, including diabetes, ischemic heart disease, hypertensive, dyslipidemia, some type of cancer and renal disease ext. Sleep has been linked to metabolic dysfunction and endocrine disturbance. 21 Other studies indicate an inverse relationship between sleep period and breast cancer risk in postmenopausal women, but no such connection exists in premenopausal women 20 One potential biological basis for sleep quality impacting the risk of breast cancer development may be attributed to that the fact that poor sleep quality represents decreased melatonin levels. Melatonin, which is primarily produced by the pineal gland, has been shown to play an important role in tumour growth inhibition via antioxidation, antiangiogenesis, and immune regulation. Melatonin appears to regulate the initiation, promotion, and/or progression of cancer, implying that it has regulatory signaling functionality. 22 Melatonin’s antimitotic and antioxidant activity, as well as its immune system modulation of both cellular and humoral responses, may play a role in carcinogenesis. 21 Melatonin is also intended to play a role in apoptosis and angiogenesis; it has been shown to inhibit endothelin-1 synthesis in blood vessels by inhibiting the endothelin-converting enzyme-1, which affects vascular endothelial cell release. Furthermore, in the presence of serum estradiol, melatonin inhibits cell proliferation, reduces cancer cell metastatic capacity, and counteracts estradiol’s stimulation of growth factors promoting cancer cells’ invasiveness, a primary stimulator of tumour angiogenesis. 23
In studies investigating sleep quality and breast cancer risk, while genetic and environmental factors should not be ignored, controlling for or stratifying by these variables can enhance the understanding of their contributions. Recent findings underscore the intricate interplay between genetic predispositions, environmental exposures, and behavioral choices in shaping sleep patterns and their health implications. Genetic variations have been implicated in influencing individual differences in sleep patterns and quality. Studies have identified specific genes involved in regulating circadian rhythms, sleep architecture, and responses to environmental stressors that affect sleep quality. 24 For instance, variations in the PER3 gene have been associated with altered sleep duration and susceptibility to sleep disorders, highlighting the genetic underpinnings of sleep phenotypes. 25 Polygenic risk scores, calculated based on multiple genetic variants, have shown promise in predicting sleep disorders and associated health risks. They aggregate genetic information to provide a cumulative risk assessment for conditions influenced by sleep disturbances. 26 Environmental factors such as shift work, exposure to artificial light at night, and irregular sleep schedules significantly impact sleep quality. These factors disrupt circadian rhythms and contribute to sleep disturbances linked to increased cancer risk. 27 Adverse lifestyle choices like excessive alcohol consumption and smoking also contribute to poor sleep quality, potentially exacerbating health risks. 28 Socioeconomic status, stress levels, and access to healthcare services play crucial roles in sleep quality. Lower socioeconomic status is associated with higher prevalence of sleep disorders due to increased stress and limited resources for managing sleep disturbances. 29
This study has several strengths. Firstly, its retrospective design helps reduce recall bias in sleep duration assessment. Secondly, by drawing participants from a wide hospital population and considering key covariates, potential confounding factors were effectively addressed in statistical analyses. Thirdly, the use of the PSQI questionnaire provided comprehensive understandings into sleep quality, timing, medication use, and sleep disorders, all relevant to understanding breast cancer risk.
Conclusion
Overall, our findings suggest that there may not be a significant link between self-reported sleep duration or quality and the risk of breast cancer in this study population. However, our observations indicate a potential association between sleep characteristics and the aggressiveness of breast cancer tumors (TNBC) in women from KP’s North West region of Pakistan.” However, due to this case-control design of our study, we cannot establish a temporal relationship between sleep duration and breast cancer diagnosis. The differences observed in sleep duration might be a consequence of the breast cancer diagnosis rather than a contributing risk factor.
Limitation of the Study
Despite this limitation, our findings highlight the importance of further research into the relationship between sleep and breast cancer. Future longitudinal studies are essential to determine whether sleep duration could be a modifiable risk factor for breast cancer. Understanding this relationship could lead to improved guidelines for sleep and overall health, potentially contributing to breast cancer prevention strategies.
Acknowledgments
The authors acknowledge the lab and hospital staff for their valuable contribution in this research. The authors acknowledge the support of the study participants and the staff at IRNUM and KTH, Peshawar, for their assistance in data collection.
Appendix.
List of Abbreviations
- BC
Breast cancer
- PSQI
Pittsburgh Sleep Quality Index
- KP
Khyber Pakhtunkhwa
- TNBC
Tripple Negative Breast Cancer
- OC
Oral Contraceptive
- WHR
Waist to Hip ratio
- BMI
Body Mass Index
- HER2
Human epidermal growth factor
- HR
Hormone receptor
Footnotes
Author Contributions: HN, ZD and IK design and wrote original manuscript. HN, BH and IA did investigation. FZ, ZI and MA did formal analysis and data curation. IH and MA reviewed the final version.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
De-Identification of Patient Details: All patient details were de-identified to ensure confidentiality and privacy. No identifiable information is presented in this study.
Ethical Statement
Ethical Approval
Ethical approval was obtained from the Faculty of Nutrition Sciences, Ethics Committee and Human Studies Review Board (FNS-ECHSRB) at The University of Agriculture, Peshawar, Pakistan (approval number No. 2020-02).
Statement of Patient Consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all patients for being included in the study.
ORCID iD
Ijaz-ul Haq https://orcid.org/0000-0002-4852-2938
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.*
References
- 1.Feng Y, Spezia M, Huang S, et al. Breast cancer development and progression: risk factors, cancer stem cells, signaling pathways, genomics, and molecular pathogenesis. Genes Dis. 2018;5(2):77-106. doi: 10.1016/j.gendis.2018.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA A Cancer J Clin. 2011;61(2):69-90. doi: 10.3322/caac.20107 [DOI] [PubMed] [Google Scholar]
- 3.Garcia M, Jemal AW, Ward EM, et al. Global Cancer Facts & Figures 2007. Atlanta, GA: American Cancer Society; 2007. [Google Scholar]
- 4.Bin YS, Marshall NS, Glozier N. Secular trends in adult sleep duration: a systematic review. Sleep Med Rev. 2012;16(3):223-230. doi: 10.1016/j.smrv.2011.05.001 [DOI] [PubMed] [Google Scholar]
- 5.Pires ML, Benedito-Silva AA, Mello MT, Pompeia SDGC, Tufik S. Sleep habits and complaints of adults in the city of São Paulo, Brazil, in 1987 and 1995. Braz J Med Biol Res. 2007;40:1505-1515. [PubMed] [Google Scholar]
- 6.Marshall NS, Glozier N, Grunstein RR. Is sleep duration related to obesity? A critical review of the epidemiological evidence. Sleep Med Rev. 2008;12(4):289-298. doi: 10.1016/j.smrv.2008.03.001 [DOI] [PubMed] [Google Scholar]
- 7.Stevens RG, Blask DE, Brainard GC, et al. Meeting report: the role of environmental lighting and circadian disruption in cancer and other diseases. Environ Health Perspect. 2007;115(9):1357-1362. doi: 10.1289/ehp.10200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verkasalo PK, Lillberg K, Stevens RG, et al. Sleep duration and breast cancer: a prospective cohort study. Cancer Res. 2005;65(20):9595-9600. doi: 10.1158/0008-5472.CAN-05-2138 [DOI] [PubMed] [Google Scholar]
- 9.Schernhammer ES, Schulmeister K. Melatonin and cancer risk: does light at night compromise physiologic cancer protection by lowering serum melatonin levels? Br J Cancer. 2004;90(5):941-943. doi: 10.1038/sj.bjc.6601626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147:573-577. [DOI] [PubMed] [Google Scholar]
- 11.Lwanga SK, Lemeshow S, World Health Organization . Sample Size Determination in Health Studies: A Practical Manual. Geneva, Switzerland: World Health Organization; 1991. [Google Scholar]
- 12.World Health Organization (WHO) . Obesity: Preventing and Managing the Global Epidemic. Report of a WHO Consultation. Geneva, Switzerland: WHO; 1998. (WHO/NT/98.1). [PubMed] [Google Scholar]
- 13.World Health Organization . Waist Circumference and Waist-Hip Ratio: Report of a WHO Expert Consultation. Geneva, Switzerland: WHO; 2011. [Google Scholar]
- 14.Xiao Q, Signorello LB, Brinton LA, Cohen SS, Blot WJ, Matthews CE. Sleep duration and breast cancer risk among black and white women. Sleep Med. 2016;20:25-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacob L, Scholten PC, Kostev K, Kalder M. Association between sleep disorders and the presence of breast cancer metastases in gynecological practices in Germany: a case–control study of 11,412 women. Breast Cancer Res Treat. 2018;171:443-448. [DOI] [PubMed] [Google Scholar]
- 16.Zaki NF, Sabri YM, Farouk O, et al. Depressive symptoms, sleep profiles and serum melatonin levels in a sample of breast cancer patients. Nat Sci Sleep. 2020;12:135-149, doi: 10.2147/NSS.S246947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu C, Sun H, Huang J, et al. Long-term sleep duration as a risk factor for breast cancer: evidence from a systematic review and dose-response meta-analysis. BioMed Res Int. 2017;2017;1-11. doi: 10.1155/2017/3476719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vaughn C, Thompson CL, Millen AE, et al. Sleep quality, duration, and breast cancer aggressiveness. Breast Cancer Res Treat. 2017;164(1):169-178. doi: 10.1007/s10549-017-4242-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Girschik J, Heyworth J, Fritschi L. Self-reported sleep duration, sleep quality, and breast cancer risk in a population-based case-control study. Am J Epidemiol. 2013;177(4):316-327. doi: 10.1093/aje/kws402 [DOI] [PubMed] [Google Scholar]
- 20.Khawaja A, Rao S, Li L, Thompson CL. Sleep duration and breast cancer phenotype. J Cancer Epidemiol. 2013;2013:467927. doi: 10.1155/2013/467927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cao J, Eshak ES, Liu K, et al. Sleep duration and risk of breast cancer: the JACC Study. Breast Cancer Res Treat. 2019;174(1):219-225. doi: 10.1007/s10549-018-0503-1 [DOI] [PubMed] [Google Scholar]
- 22.Mayo JC, Cernuda R, Quiros I, et al. Understanding the role of melatonin in cancer metabolism. Melatonin Res. 2019;2:76-104. [Google Scholar]
- 23.Kubatka P, Zubor P, Busselberg D, et al. Melatonin and breast cancer: evidences from preclinical and human studies. Crit Rev Oncol Hematol. 2018;122:133-143. doi: 10.1016/j.critrevonc.2017.12.011 [DOI] [PubMed] [Google Scholar]
- 24.Dashti HS. Genome-wide association study identifies genetic loci for self-reported habitual sleep duration supported by accelerometer-derived estimates. Nat Commun. 2015;6:6355. doi: 10.1038/ncomms7355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johansson C. A PERIOD3 variant causes a circadian phenotype and is associated with a seasonal mood trait and working memory. Am J Med Genet Part B: Neuropsychiatric Genetics 2016;171(7):947-955. doi: 10.1002/ajmg.b.32465 [DOI] [Google Scholar]
- 26.Lane JM, Liang J, Vlasac I, et al. Genome-wide association analyses of sleep disturbance traits identify new loci and highlight shared genetics with neuropsychiatric and metabolic traits. Nat Genet. 2017;49(2):274-281. doi: 10.1038/ng.3749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schernhammer ES, Laden F, Speizer FE, et al. Night-shift work and risk of colorectal cancer in the nurses’ health study. J Natl Cancer Inst. 2003;95(11):825-828. doi: 10.1093/jnci/95.11.825 [DOI] [PubMed] [Google Scholar]
- 28.Grandner MA. Sleep duration, cardiovascular disease, and proinflammatory biomarkers. Nat Sci Sleep. 2010;2:213-224. doi: 10.2147/NSS.S10207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grandner MA. Socioeconomic disparities in sleep: contribution of individual-level factors and neighborhood characteristics. Sleep Med. 2016;18:27-35. doi: 10.1016/j.sleep.2015.01.013 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.*