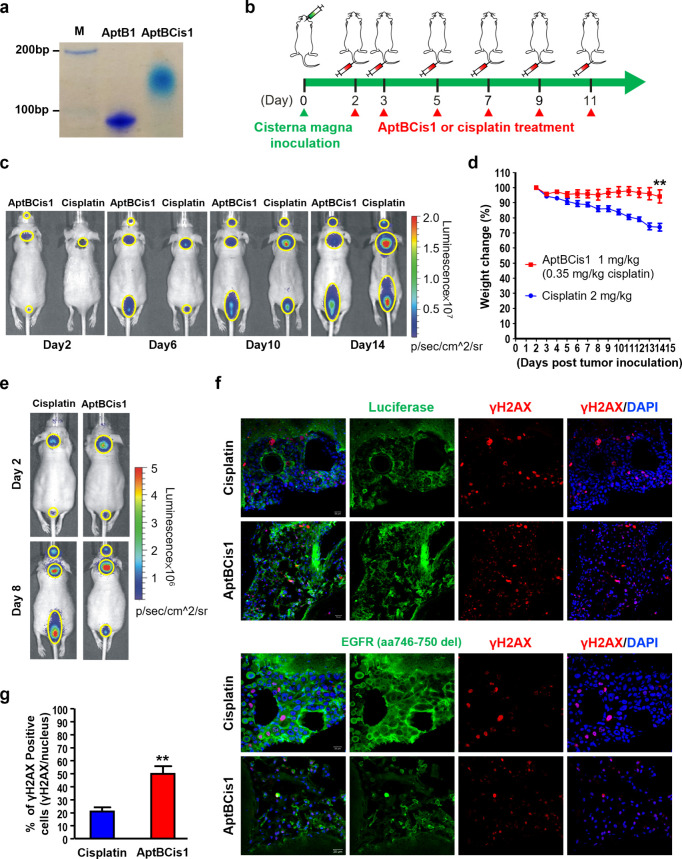
Figure 4.
AptBCis1, an aptamer–cisplatin conjugate, showed antitumor effects in lung cancer LM diseases. (a) The native polyacrylamide gel electrophoresis result showed successful conjugation of the AptB1 and the cisplatin. (b) The scheme illustrated the timeline of tumor cell inoculation and IV drug treatment (AptBCis1 or cisplatin). (c) The mice were monitored by IVIS; BLI signal intensity implicated corresponding tumor burden. Mice in the cisplatin group showed stronger BLI signals than mice in the AptBCis1 group on Day 14 post tumor inoculation (n = 8 in each group). The lesions are outlined with yellow circles. (d) Significant body weight reduction in the cisplatin group (**P < 0.01). Formulation of body weight change: (Day X/Day 2) %. (e) The IVIS images were taken at Day 2 and Day 8 post tumor inoculation; cisplatin or AptBCis1 was given on Days 2, 3, 5, and 7 post tumor inoculation. The mice were sacrificed on Day 8, and the brains were subjected to immunofluorescent studies. The lesions are outlined with yellow circles. (f) The confocal microscopy images showed better preserved tumor cell contours (green; upper panel: luciferase; lower panel: EGFR) and lower percentage of γH2AX-positive cells (red) in the cisplatin group. (g) A lower percentage of γH2AX-positive cells was observed in the cisplatin group. A total of 1200 or 450 cells, respectively, was analyzed in the cisplatin or the AptBCis1 group. Asterisks denote statistically significant differences. **P < 0.01 (unpaired t test).