Abstract
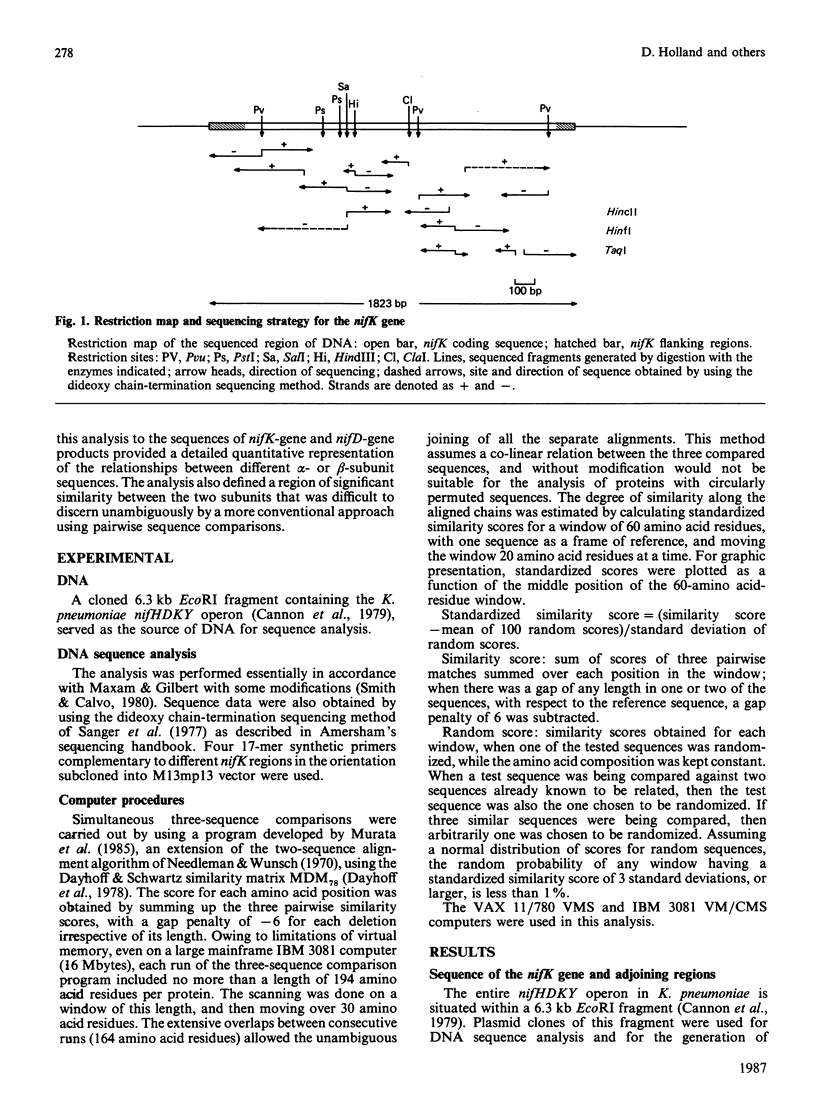
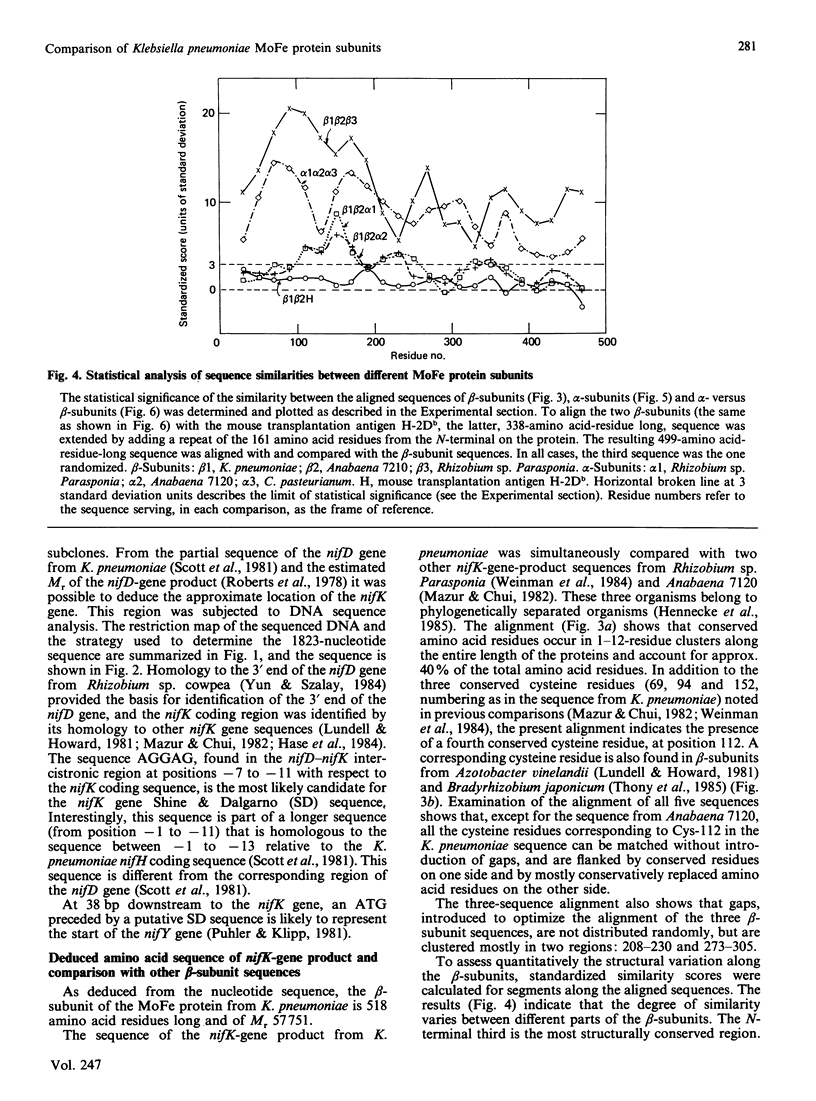
The nucleotide sequence was determined for part of the Klebsiella pneumoniae nif gene cluster containing the 3' end of the nifD gene and the entire length of the nifK gene (encoding the alpha- and beta-subunits of the nitrogenase MoFe protein respectively), as well as the putative start of the nifY gene, a gene of as yet unknown function. A broad-based comparison of a number of MoFe protein alpha-subunits, beta-subunits and alpha-versus beta-subunits was carried out by the use of a computer program that simultaneously aligns three protein sequences according to the mutation data matrix of Dayhoff. A new kind of quantitative statistical measure of the similarity between the aligned sequences was obtained by calculating and plotting standardized similarity scores for overlapping segments along the aligned proteins. This calculation determines if a test sequence is similar to the consensus sequence of two other proteins that are known to be related to each other. The different beta-subunits compared were found to be significantly similar along most of their sequence, with the exception of two relatively short regions centred around residues 225 and 300, which contain insertions/deletions. The overall pattern of similarity between different alpha-subunits exhibits resemblance to the overall pattern of similarity between different beta-subunits, including regions of low similarity centred around residues 225 and 340. Comparison of alpha-subunits with beta-subunits showed that a region of significant similarity between the two types of subunits was located approximately between residues 120 and 180 in both subunits, but other parts of the proteins were only marginally similar. These results provide insights into likely tertiary structural features of the MoFe protein subunits.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cannon F. C., Riedel G. E., Ausubel F. M. Overlapping sequences of Klebsiella pneumoniae nifDNA cloned and characterized. Mol Gen Genet. 1979 Jul 2;174(1):59–66. doi: 10.1007/BF00433306. [DOI] [PubMed] [Google Scholar]
- Cannon M., Hill S., Kavanaugh E., Cannon F. A molecular genetic study of nif expression in Klebsiella pneumoniae at the level of transcription, translation and nitrogenase activity. Mol Gen Genet. 1985;198(2):198–206. doi: 10.1007/BF00382996. [DOI] [PubMed] [Google Scholar]
- Colman P. M., Varghese J. N., Laver W. G. Structure of the catalytic and antigenic sites in influenza virus neuraminidase. Nature. 1983 May 5;303(5912):41–44. doi: 10.1038/303041a0. [DOI] [PubMed] [Google Scholar]
- Golden J. W., Robinson S. J., Haselkorn R. Rearrangement of nitrogen fixation genes during heterocyst differentiation in the cyanobacterium Anabaena. Nature. 1985 Apr 4;314(6010):419–423. doi: 10.1038/314419a0. [DOI] [PubMed] [Google Scholar]
- Hase T., Wakabayashi S., Nakano T., Zumft W. G., Matsubara H. Structural homologies between the amino acid sequence of Clostridium pasteurianum MoFe protein and the DNA sequences of nifD and K genes of phylogenetically diverse bacteria. FEBS Lett. 1984 Jan 23;166(1):39–43. doi: 10.1016/0014-5793(84)80040-x. [DOI] [PubMed] [Google Scholar]
- Lammers P. J., Haselkorn R. Sequence of the nifD gene coding for the alpha subunit of dinitrogenase from the cyanobacterium Anabaena. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4723–4727. doi: 10.1073/pnas.80.15.4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundell D. J., Howard J. B. Isolation and sequences of the cysteinyl tryptic peptides from the MoFe-protein of Azotobacter vinelandii nitrogenase. J Biol Chem. 1981 Jun 25;256(12):6385–6391. [PubMed] [Google Scholar]
- MacNeil T., MacNeil D., Roberts G. P., Supiano M. A., Brill W. J. Fine-structure mapping and complementation analysis of nif (nitrogen fixation) genes in Klebsiella pneumoniae. J Bacteriol. 1978 Oct;136(1):253–266. doi: 10.1128/jb.136.1.253-266.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur B. J., Chui C. F. Sequence of the gene coding for the beta-subunit of dinitrogenase from the blue-green alga Anabaena. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6782–6786. doi: 10.1073/pnas.79.22.6782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrick M., Filser M., Dixon R., Elmerich C., Sibold L., Houmard J. The use of translocatable genetic elements to construct a fine-structure map of the Klebsiella pneumoniae nitrogen fixation (nif) gene cluster. J Gen Microbiol. 1980 Apr;117(2):509–520. doi: 10.1099/00221287-117-2-509. [DOI] [PubMed] [Google Scholar]
- Mortenson L. E., Thorneley R. N. Structure and function of nitrogenase. Annu Rev Biochem. 1979;48:387–418. doi: 10.1146/annurev.bi.48.070179.002131. [DOI] [PubMed] [Google Scholar]
- Murata M., Richardson J. S., Sussman J. L. Simultaneous comparison of three protein sequences. Proc Natl Acad Sci U S A. 1985 May;82(10):3073–3077. doi: 10.1073/pnas.82.10.3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needleman S. B., Wunsch C. D. A general method applicable to the search for similarities in the amino acid sequence of two proteins. J Mol Biol. 1970 Mar;48(3):443–453. doi: 10.1016/0022-2836(70)90057-4. [DOI] [PubMed] [Google Scholar]
- Reyes A. A., Schöld M., Wallace R. B. The complete amino acid sequence of the murine transplantation antigen H-2Db as deduced by molecular cloning. Immunogenetics. 1982;16(1):1–9. doi: 10.1007/BF00364437. [DOI] [PubMed] [Google Scholar]
- Roberts G. P., MacNeil T., MacNeil D., Brill W. J. Regulation and characterization of protein products coded by the nif (nitrogen fixation) genes of Klebsiella pneumoniae. J Bacteriol. 1978 Oct;136(1):267–279. doi: 10.1128/jb.136.1.267-279.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott K. F., Rolfe B. G., Shine J. Biological nitrogen fixation: primary structure of the Klebsiella pneumoniae nifH and nifD genes. J Mol Appl Genet. 1981;1(1):71–81. [PubMed] [Google Scholar]
- Segal D. M., Padlan E. A., Cohen G. H., Rudikoff S., Potter M., Davies D. R. The three-dimensional structure of a phosphorylcholine-binding mouse immunoglobulin Fab and the nature of the antigen binding site. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4298–4302. doi: 10.1073/pnas.71.11.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. R., Calvo J. M. Nucleotide sequence of the E coli gene coding for dihydrofolate reductase. Nucleic Acids Res. 1980 May 24;8(10):2255–2274. doi: 10.1093/nar/8.10.2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinman J. J., Fellows F. F., Gresshoff P. M., Shine J., Scott K. F. Structural analysis of the genes encoding the molybdenum-iron protein of nitrogenase in the Parasponia rhizobium strain ANU289. Nucleic Acids Res. 1984 Nov 26;12(22):8329–8344. doi: 10.1093/nar/12.22.8329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T. T., Kabat E. A. An analysis of the sequences of the variable regions of Bence Jones proteins and myeloma light chains and their implications for antibody complementarity. J Exp Med. 1970 Aug 1;132(2):211–250. doi: 10.1084/jem.132.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamane T., Weininger M. S., Mortenson L. E., Rossmann M. G. Molecular symmetry of the MoFe protein of nitrogenase. Structural homology/nitrogen fixation/x-ray crystallography. J Biol Chem. 1982 Feb 10;257(3):1221–1223. [PubMed] [Google Scholar]
- Yun A. C., Szalay A. A. Structural genes of dinitrogenase and dinitrogenase reductase are transcribed from two separate promoters in the broad host range cowpea Rhizobium strain IRc78. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7358–7362. doi: 10.1073/pnas.81.23.7358. [DOI] [PMC free article] [PubMed] [Google Scholar]


