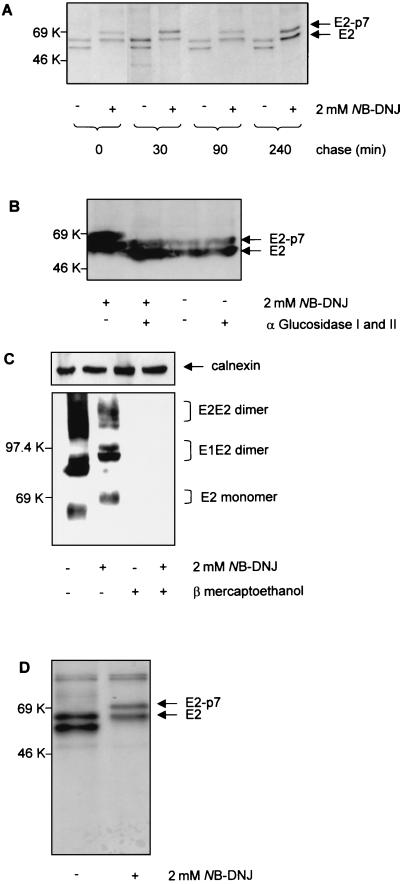
FIG. 4.
Biosynthesis and processing of BVDV envelope glycoproteins in the presence of NB-DNJ. MDBK cells were infected with BVDV at an MOI of 1. (A) Eighteen hours p.i., the cells were treated (+) or not treated (−) with 2 mM NB-DNJ. Two hours later, the cells were pulse-labeled with [35S]methionine and [35S]cysteine for 15 min, chased for the times indicated in the continuous presence of the drug, and immunoprecipitated with MAb 214. The proteins were analyzed by SDS–10% PAGE under reducing conditions and visualized by autoradiography. (B) Infected cells were grown for 18 h in the absence (−) or presence (+) of 2 mM NB-DNJ. Cell lysates were analyzed for protein content, and the equivalent of 20 μg of protein was digested (+) or not digested (−) with a mixture of α-glucosidases I and II. The proteins were separated by SDS–10% PAGE under reducing conditions and analyzed with a Western blot stained with MAb 214. (C) Infected cells were grown for 18 h in the absence (−) or presence (+) of 2 mM NB-DNJ. Cell lysates were boiled for 5 min in the absence (−) or presence (+) of 5% β-mercaptoethanol prior to separation by SDS–10% PAGE. The proteins were analyzed with a Western blot stained with MAb 158. Calnexin was detected with an anticalnexin antibody and used as loading control. (D) Conditions were the same as described for panel A, except that infected cells were lysed immediately after the 15 min of pulse and immunoprecipitated with MAb 158.