Abstract
Purpose
To evaluate the use, acceptability, and experience of a seven-item palliative care referral screening tool in an outpatient oncology setting.
Methods
A two-phase convergent parallel mixed-methods study. Patient participants who met any of the “Royal Marsden Triggers Tool” criteria were compared with those who did not in terms of demographic data, palliative care needs (Integrated Palliative Outcome Scale, IPOS) and quality of life indicators (EORTC-QLQ-C30).
In-depth interviews were carried out with patients and oncology staff about their views and experience of the “Royal Marsden Triggers Tool”. Qualitative and quantitative data were triangulated at data interpretation.
Results
Three hundred forty-eight patients were recruited to the quantitative phase of the study of whom 53% met at least one of the Triggers tool palliative care referral criteria. When compared with patients who were negative using the Triggers tool, “Royal Marsden Triggers Tool” positive patients had a lower quality of life (EORTC QLQ-C30 Global Health Status scale (p < 0.01)) and a higher proportion had severe or overwhelming physical needs on IPOS (38% versus 20%, p < 0.001). Median survival of “Royal Marsden Triggers Tool” positive patients was 11.7 months.
Sixteen staff and 19 patients participated in qualitative interviews. The use of the tool normalised palliative care involvement, supporting individualised care and access to appropriate expertise.
Conclusion
The use of a palliative care referral tool streamlines palliative care within oncology outpatient services and supports teams working together to provide an early holistic patient-centred service. Further research is needed to evaluate the effectiveness and feasibility of this approach.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00520-024-08921-5.
Keywords: Normalisation, Holistic, Integrated Palliative Outcome Scale (IPOS), EORTC-QLQ-C30, Early palliative care, Palliative care referral tool
Introduction
The outpatient oncology setting provides an ideal opportunity to deliver early integrated palliative care through the proactive identification of needs, timely intervention and crisis prevention [1, 2].
A major challenge faced by palliative care as a specialty is how to address the increased resource requirements as palliative care is no longer confined to end of life care [3, 4]. There is a need to prioritise the availability of existing specialist palliative care resources for those who would benefit most [5–7].
International bodies have called for the development of sustainable systems to streamline palliative care referrals, to normalise palliative care involvement alongside oncology intervention and to ensure proactive rather than reactive identification of patients with or at risk of unmet palliative care needs [8, 9]. The use of defined palliative care referral criteria has the potential to support standardised care pathways, reduce inequitable access and triage patients who are most likely to benefit [7, 10].
Several referral criteria or palliative care screening tools have been developed for use in oncology outpatient clinics [11–14], but none have been implemented widely in clinical practice. We have been using a locally developed set of criteria, the “Royal Marsden Triggers Tool” in the oncology outpatient setting since 2017. This seven-item checklist includes patient and disease-related factors which may indicate progressive disease and increased palliative care needs [15, 16]. It was originally devised through literature review and expert consensus [17]. In retrospective evaluations, this tool has been shown to appropriately identify patients who may have benefited from palliative care before inpatient hospital death. A similar set of 11 major palliative care referral criteria were identified through a Delphi process by a panel of international palliative care experts [18]. Although these “Delphi Study Criteria” have not been validated, in a retrospective study, this approach has been shown to appropriately identify patients who were referred to a supportive care clinic [19].
The aims of this study were to (1) evaluate the sensitivity of the “Royal Marsden Triggers Tool” in identifying patients with palliative care needs in an outpatient oncology setting, (2) explore the acceptability of the “Royal Marsden Triggers Tool” to oncology staff and (3) to examine the staff and patient experience of the early palliative care service based on the use of the “Royal Marsden Triggers Tool”. To add context to this study, we also included descriptive metrics to evaluate the operationalisation of the “Delphi Study Criteria” as a secondary outcome in the study.
Methods
Study design and participants
This was a two-phase convergent parallel mixed-methods study in which quantitative and qualitative data were equally prioritised, collected independently and analysed separately prior to integration and interpretation [20]. Phase one quantitative cross-sectional data were collected at a single time point. Phase two involved qualitative in-depth interviews with patients and oncology healthcare professionals. Reporting followed GRAMMS criteria for mixed-methods studies [21].
Participants to this study were recruited from a single tertiary referral cancer centre situated across two sites in a large metropolitan setting.
Eligibility for quantitative phase 1
Adults attending an oncology clinic with a primary diagnosis of lung or upper gastro-intestinal (UGI) cancers, or sarcoma, who had not been seen by a palliative care service within the previous 3 months were eligible.
Eligibility for qualitative phase 2
Oncology healthcare professionals (doctors and nurses) working in the lung, UGI or sarcoma clinics where the “Royal Marsden Triggers Tool” was used in clinical practice and patients who were attending these oncology clinics and who met at least one of the “Royal Marsden Triggers Tool” palliative care referral criteria were invited to participate in semi-structured interviews.
Patients with communication/language needs were offered the use of interpreters, with the IPOS translated into other languages (where available and validated). However, inclusion in the in-depth interviews was limited by patients’ fluency in English.
Outcome measurements and data collection
Quantitative phase 1
Demographic data were collected from the electronic hospital medical record including clinical and patient specific data, age, gender, comorbidities, tumour diagnosis, and presence of metastases. Date of death was also recorded for those who had died within the time period between recruitment and 12 months after the last participant was recruited.
Study measures completed by the oncology clinical team based on their usual oncology clinic review included the following:
-
o
Performance status (ECOG (Eastern Co-operative Oncology Group) and Australia-modified Karnofsky Performance Status (AKPS))
- o
- o
Table 2.
Palliative care referral criteria
| Total N (%) N = 348 |
|
|---|---|
| “Royal Marsden Triggers Tool” | |
| Metastatic cancer progressing after first line treatment | 115 (33) |
| Performance status ECOG 2 and deteriorating | 32 (9.2) |
| Acute oncology or unplanned admission | 17 (4.9) |
| Severe or overwhelming symptoms | 29 (8.3) |
| Anorexia, hypercalcaemia or any effusion | 39 (11.2) |
| Moderate or severe psychological or existential distress | 20 (5.7) |
| Complex social issues | 15 (4.3) |
| Royal Marsden Triggers Tool positive for at least 1 item | 183 (53) |
| “Delphi Study Criteria” | |
| Severe physical symptoms | 35 (10.1) |
| Severe emotional symptoms | 13 (3.7) |
| Request for hastened death | 0 |
| Spiritual or existential crisis | 1 (0.3) |
| Assistance with decision-making/care plan | 4 (1.1) |
| Patient request | 2 (0.6) |
| Delirium | 0 |
| Brain/leptomeningeal metastases | 14 (4) |
| Spinal cord compression/cauda equina | 2 (0.6) |
| ≤ 3-month diagnosis of advanced/incurable cancer with median survival ≤ 1 year | 57 (16.4) |
| Diagnosis of advanced cancer with progressive disease despite 2nd line systemic therapy (incurable) | 43 (12.4) |
| Delphi Study Criteria positive for at least 1 item | 133 (38.2) |
Staff recorded which criteria on each tool were met during that clinic visit.
Patient participants completed the following:
-
o
Integrated Palliative Outcome Scale (IPOS): a validated holistic needs assessment tool used widely in clinical practice to determine patients’ palliative care needs and priorities of care which are scored using a Likert scale 0–4 with numerical and descriptive response anchors [22]
-
o
EORTC-QLQ-C30 quality of life measures: a 30-item validated questionnaire developed to assess the quality of life of cancer patients [23]
Qualitative phase 2
The qualitative semi-structured interviews were carried out by a researcher trained in this approach (LK), face to face or over the phone, as per participant preference/relevant social distancing guidelines. An interview topic guide (Supplementary Tables 1 and 2) was developed with public and patient involvement (see section at the end of the manuscript) based on available literature and revised as the study progressed to allow exploration of new and emerging themes.
Staff participants were interviewed about their experience of using the “Royal Marsden Triggers Tool” and the palliative care service embedded within the oncology clinic based on the tool.
Patient participants were interviewed about their views and experience of the embedded palliative care service in the oncology clinics where the “Royal Marsden Triggers Tool” was used.
Data analysis and sample size
Quantitative phase 1
The demographics of the total study population were described. Patient participants were categorised into cohorts according to whether they met the criteria for palliative care referral. Participants who scored positive on any item on the “Royal Marsden Triggers Tool” or “Delphi Study Criteria” were defined as being “Royal Marsden Triggers Tool positive” or “Delphi Study Criteria positive”. Patients scoring positive and negative using each of the tools were compared in terms of demographic data, performance status, palliative care needs (IPOS) and quality of life indicators (EORTC-QLQ-C30).
Descriptive analysis methods were used to summarise the study data including mean/median and standard deviation/interquartile range for continuous data and frequency with percentages for categorical data. Data were compared between referral tool positive and negative cohorts using t-test/Mann–Whitney and chi-square/Fishers exact test as appropriate. Data from participants who were still alive when the death data were collected (12 months after last participant recruited) were censored at this date. Survival time (between recruitment and death) was compared between cohorts using the log-rank test method.
Missing IPOS data items were excluded from IPOS item analysis of palliative care needs. All study participants with both clinician assessment using the “Royal Marsden Triggers Tool” and completed IPOS study questionnaire were included in the primary endpoint analysis of the sensitivity of the “Royal Marsden Triggers Tool” in identifying cancer patients with palliative care needs. In this study, in the absence of validated method of how to define a patient with palliative care needs, the predetermined reference standard for patients with palliative care needs was defined as an IPOS score of 2 (moderate), 3 (severe) or 4 (overwhelming/always) on any item [24]. Binary tables were devised to calculate the sensitivity, specificity, positive and negative predictive values, and overall accuracy of the “Royal Marsden Triggers Tool”.
The proportion of participants scoring 3 or 4 on each IPOS item was calculated to define those with “severe” or “overwhelming” needs.
To observe a target “Royal Marsden Triggers Tool” sensitivity of 80% (alternative hypothesis Ha) from an unacceptable 60% (null hypothesis H0) sensitivity, based on two-sided 5% alpha and 80% power, and an estimated prevalence of palliative care needs ranging from 40 to 90%, a minimum of 112 patients were required from each tumour group, with a total estimated minimum required sample size of 336 (Supplementary Table 3) [25].
Secondary objectives included evaluation of how the “Delphi Study Criteria” performed in clinical practice in terms of identifying patients with palliative care needs and association of both the referral tools with measures of quality-of-life (EORTC-QLQ-C30). This study was not designed or powered for direct comparative analyses between the “Royal Marsden Triggers Tool” and “Delphi Study Criteria”.
The EORTC-QLQ-C30 functional and Global Health Status Scales were calculated and described according to the scoring manual using mean and standard deviation and median and interquartile range [26].
Qualitative phase 2
A purposive, theoretical sampling technique was adopted, ensuring that not only a range of ages/tumour variations/gender/clinical experience and professions were reflected but could also test emerging theory and sample accordingly until reaching data saturation.
In keeping with a modified Grounded Theory approach [27], data collection and analysis occurred simultaneously. Digitally recorded and verbatim interview transcripts were analysed alongside original recordings and coded independently by two researchers (LK and AMS). Coding followed an iterative open, axial, selective coding structure, with constant comparative technique. Deeper analyses of the interview data and the development and refinement of the codes into themes and subthemes were discussed in regular study management group meetings (LK, AMS, and JD with input from TW, MP, and NP) and were tested in an iterative process in subsequent interviews. Data saturation was reached when no new themes were generated.
After both the quantitative and qualitative data were analysed separately, the findings were triangulated during interpretation of the overall study findings. The data were examined together to identify areas of agreement (convergence), dissonance (contradiction) and complementarity [28].
Results
Quantitative phase 1
A total of 578 patients were screened between 3rd December 2018 and 20th August 2020, of whom 436 patients were eligible and invited to participate in the study. Of these, 348 patients were recruited.
Participant characteristics
The clinical characteristics and demographics for the study cohort are presented in Table 1. The mean age was 66 years, and 98% (341/348) of the patients had an ECOG performance status between 0 and 2, reflecting their ability for self-care. Eighty-one percent (282/348) of the patients had metastatic disease and 59% (205/348) had one or more comorbidities.
Table 1.
Demographics, clinical characteristics, and death data
| “Royal Marsden Triggers Tool” | “Delphi Study Criteria” | ||||
|---|---|---|---|---|---|
| Total study population N = 348 |
Trigger negative N = 165 |
Trigger positive N = 183 |
Delphi negative N = 215 |
Delphi positive N = 133 |
|
| Age (years), mean (standard deviation) | 66.0 (11.4) | 66.9 (10.7) | 65.2 (12.0) | 66.3 (11.4) | 65.6 (11.6) |
| First oncology clinic to recruitment (months), median (range) | 1.7 (0–170.3) | 1.4 (0–153.7) | 1.97 (0–170.3) | 1.6 (0–153.7) | 1.7 (0–170.3) |
| Diagnosis to recruitment¥ (months), median (range) | 11.4 (0.07–443.2) | 5.5 (0.07–443.2)*** | 16.5 (0.07–201.9)*** | 11.3 (0.07–179.9) | 11.5 (0.07–443.2) |
| Comorbidities N (%) | 205 (59) | 102 (61.8) | 103 (56.3) | 126 (58.6) | 79 (59.4) |
| ECOG performance status¥¥ 0, 1, 2 N (%) | 341 (98) | 163 (99) | 178 (97) | 214 (99.6) | 127 (95.5) |
| Australian Karnofsky Performance Status ≥ 70 N (%) | 341 (98) | 164 (99.4) | 177 (96.7) | 214 (99.5) | 127 (95.5) |
| Presence of metastases N (%) | 282 (81) | 119 (72.1)*** | 163 (89.1)*** | 164 (76.3)* | 118 (88.7)* |
| Median survival months (95% CI) | 17.1 (16–18.7) | 20.8 (18.9–NR)** | 11.7 (9.7–15.6)** | 20.5 (17.9–NR)** | 10.4 (8.8–13.8)** |
NR not reached (median survival)
*p < 0.01
**p < 0.001
***p < 0.0001
¥As a tertiary referral cancer centre, many patients are diagnosed elsewhere before starting treatment at the centre
¥¥No patients had an ECOG performance status of 0
There was no difference between “Royal Marsden Triggers Tool” positive and negative cohorts in terms of age, comorbidities, or performance status. A higher proportion of “Royal Marsden Triggers Tool” positive participants had metastatic disease compared with negative participants (89.1% versus 72.1%, p < 0.0001). The results were similar when the “Delphi Study Criteria” positive and negative cohorts were compared.
Patients who scored positive on either the “Royal Marsden Triggers Tool” or the “Delphi Study Criteria” had a lower median survival (log rank < 0.001).
The “Royal Marsden Triggers Tool” and “Delphi Study Criteria”
52.6% (183/348) and 38.2% (133/348) of the total study participants met at least one of the referral criteria according to the “Royal Marsden Triggers Tool” and “Delphi Study Criteria” respectively (Table 2).
Palliative care needs of study population
Of the 348 patients recruited to the study, 9 patients did not complete the IPOS assessment. Of the remaining 339 patients, 0.3% of IPOS data were missing.
Ninety-one percent (308/339) of the total participants scored 2 (moderate), 3 (severe) or 4 (overwhelming/always) on at least one IPOS item. There was no difference between “Royal Marsden Triggers Tool” positive and negative patients (91% and 90% respectively). The sensitivity of the “Royal Marsden Triggers Tool”, i.e. the ability to correctly identify patients with palliative care needs as defined in this way, was 54% (95% CI 48–61%). The sensitivity of the “Delphi Study Criteria” to identify patients with at least one IPOS item of 2, 3 or 4 in severity was 42% (95% CI 36–47%) (Supplementary Table 4).
Many participants (76.1% (258/339)) had at least one IPOS item which scored either 3 (severe) or 4 (overwhelming/always) (Table 3). A higher proportion of patients who were positive for the “Royal Marsden Triggers Tool” had at least one severe or overwhelming physical need (38% (69/182) versus 20% (32/157) for those who were negative, chi2 12.4, p < 0.001, sensitivity 68% (95% CI 58–77%)). A higher proportion of these had pain, shortness of breath, weakness/lack of energy, constipation, poor appetite, and poor mobility. “Royal Marsden Triggers Tool” positive participants had a lower quality of life on the EORTC QLQ–C30 Global Health Status scale than those who were negative (p < 0.01). They also had lower levels of physical, role and social functioning. A similar pattern of difference was seen with the “Delphi Study Criteria” (Table 3).
Table 3.
Patient-reported concerns and issues using IPOS and EORTC QLQ-C30¥ scores
| Total (N = 339), N (%) | “Royal Marsden Triggers Tool” negative (N = 157), N (%) | “Royal Marsden Triggers Tool” positive (N = 182), N (%) | “Delphi Study Criteria” negative (N = 206), N (%) | “Delphi Study Criteria” positive (N = 133), N (%) | |
|---|---|---|---|---|---|
| IPOS scores of 3 or 4 in severity | |||||
| Physical symptoms | |||||
| Pain | 26 (7.7) | 6 (3.8)* | 20 (11)* | 8 (3.9)** | 18 (13.5)** |
| Shortness of breath | 21 (6.2) | 3 (1.9)* | 18 (9.9)* | 7 (3.4)** | 14 (10.5)** |
| Weakness/lack of energy | 38 (11.3) | 10 (6.5)** | 28 (15.5)** | 15 (7.4)** | 23 (17.4)** |
| Nausea | 10 (2.9) | 3 (1.9) | 7 (3.8) | 5 (2.4) | 5 (3.8) |
| Vomiting | 3 (0.9) | 1 (0.6) | 2 (1.1) | 1 (0.5) | 2 (1.5) |
| Poor appetite | 29 (8.6) | 7 (4.5)* | 22 (12.1)* | 12 (5.8)* | 17 (12.8)* |
| Constipation | 23 (6.8) | 6 (3.8)* | 17 (9.3)* | 8 (3.9)** | 15 (11.3)** |
| Sore or dry mouth | 15 (4.4) | 4 (2.6) | 11 (6) | 5 (2.4)* | 10 (7.5)* |
| Drowsiness | 12 (3.6) | 4 (2.6) | 8 (4.4) | 7 (3.4) | 5 (3.8) |
| Poor mobility | 26 (7.7) | 7 (4.5)* | 19 (10.4)* | 11 (5.4)* | 15 (11.3)* |
| Emotional symptoms | |||||
| Patient anxiety | 78 (23.1) | 35 (22.3) | 43 (23.8) | 40 (19.4) | 38 (28.8) |
| Family anxiety | 132 (39.1) | 64 (40.8) | 68 (37.6) | 72 (35) | 60 (45.5) |
| Depression | 28 (8.3) | 12 (7.7) | 16 (8.8) | 14 (6.8) | 14 (10.6) |
| Feeling at peace | 71 (21.1) | 37 (23.7) | 34 (18.8) | 48 (23.4) | 23 (17.4) |
| Communication/practical issues | |||||
| Sharing feelings | 112 (33.2) | 51 (32.7) | 61 (33.7) | 60 (29.3) | 52 (39.4) |
| Information | 136 (40.2) | 69 (43.9) | 67 (37) | 78 (37.9) | 58 (43.9) |
| Practical problems | 36 (10.7) | 23 (14.6)* | 13 (7.2)* | 22 (10.7) | 14 (10.6) |
| EOTRC QLQ-C30 scores | |||||
| Global health status/quality of life | |||||
| Mean (SD) | 67.2 (21.4)** | 60.3 (23.8)** | 67 (21.6)*** | 58.2 (24)*** | |
| 95% CI | (63.8–70.6) | (56.9–63.9) | (64–69.9) | (54.1–62.3) | |
| Physical functioning | |||||
| Mean (SD) | 78.8 (17.7)*** | 69.8 (25.3)*** | 78.2 (20.1)*** | 67.5 (24.6)*** | |
| 95% CI | (76–81.6) | (66.1–73.5) | (75.5–81) | (63.2–71.7) | |
| Role functioning | |||||
| Mean (SD) | 75 (26.93)*** | 61.6 (31.90)*** | 75.1 (26.4)*** | 56.8 (33)*** | |
| 95% CI | (70.7–79.2) | (56.8–66.3) | (71.5–78.7) | (51.1–62.5) | |
| Emotional functioning | |||||
| Mean (SD) | 75.8 (21.15) | 74.4 (23.48) | 77 (21.3) | 72.20 (23.9) | |
| 95% CI | (72.45–79.16) | (71–77.9) | (74.1–80) | (68.1–76.3) | |
| Cognitive functioning | |||||
| Mean (SD) | 82.9 (19.11) | 78.9 (22.27) | 83.2 (18.4)** | 77 (23.9)** | |
| 95% CI | (79.9–85.9) | (75.7–82.2) | (80.6–85.7) | (72.9–81.1) | |
| Social functioning | |||||
| Mean (SD) | 77.5 (24.7)** | 68.2 (29.1)** | 78.2 (23.9)*** | 63.89 (30.5)*** | |
| 95% CI | (73.6–81.4) | (65–72.5) | (45.88–81.43) | (58.64–69.14) |
*p < 0.05
**p < 0.01
***p < 0.001
¥A high score for the functional scales represents a high/healthy level of functioning and a high score for the global health status/quality of life scale represents a high quality of life
Qualitative phase 2
Twenty-five staff and 255 patients were screened across the three oncology groups. Sixteen staff (consultants (n = 8), oncology trainee doctors (n = 2) and clinical nurse specialists (n = 6)) working in lung (n = 9), UGI (n = 5) and sarcoma (n = 2) clinics and 19 patients (11 men, 8 women, 10 with lung cancer, 7 with UGI cancer and 2 with sarcoma) consented to participate in the interviews between December 2018 and May 2021 (Supplementary Table 5 Qualitative participant characteristics).
Healthcare professional data and then patient/family data were analysed. We then undertook further constant comparative analysis to consider the data across both staff and patients. Nine themes were derived across three categories. These are drawn together in Table 4 with a selection of representative quotes.
Table 4.
Results of qualitative data analysis from patients and staff about the “Royal Marsden Triggers Tool”: themes, subthemes and illustrative quotes
| Category | Themes | Quotes |
|---|---|---|
| Category 1: staff acceptability of the “Royal Marsden Triggers Tool” | Theme 1: more than a tool… | “It helps triage doesn’t it… it helps triage and gives them a sort of an objective idea of what’s going on… so I think they can be of value… because it helps to focus the mind on what the problems are.” (TCI0005) |
| Theme 2: perceived limitations | “…availability of the palliative care service is probably a bigger, has a bigger influence than the Trigger tool itself because if we have just someone in clinic with a tool up on the wall and ticking okay do a palliative care referral, in some of those cases they will go off to the community teams and the community teams just won’t have the capacity to usefully benefit them” (TSI0004) | |
| Category 2: staff experience of the palliative care service based on the “Royal Marsden Triggers Tool” | Theme 3: normalises palliative care for professionals | “so with Triggers obviously in lung,… I think it has really opened up that conversation about, um, palliative care” (TCI0002) |
| Theme 4: access to immediate expertise…and more | “….it is a more streamlined approach to flag a patient who has a specific… symptom control needs, that may or may not be being fully met, and then… fast track them into a quick review process with… the expert team” (TCI0006) | |
| “….a lot of communication skills…that you can even help the patient explore… some difficulties that they are having either with family, either with social support… so definitely its complementary to our practise” (TCI0001) | ||
| “it’s about symptom management and obviously trying to get people to live a great, as good a quality of life as possible with their symptoms…advance care planning…. end of life, supporting home, hospice, other options” (TSI0005) | ||
| Theme 5: supporting individualised needs | “…it may not be right for everyone to do it at (the) first meeting but then it is right for a large amount of patients and there is not going to be a right time for everyone….” (TCI0004) | |
| ….so there’s a group that should have first contact and then they know that there is somebody there and then there’s a group that probably need a bit more active engagement…” (TSI0001) | ||
| Theme 6: perceived organisational barriers | “….I think the tricky bit about clinics….the clinic room is quite small….so there’s often a lot of people then, in and out of clinic.” (TCI0003) | |
| “the problem is there ain’t enough of you for this hospital so we need more.” (TCI0005) | ||
| Category 3: patient experience of the palliative care service based on “Royal Marsden Triggers Tool” | Theme 7: integrating care between oncology and palliative care | “one of the things I see in the hospital is that it’s not a different department, all of you are a team. All of you are a team, the doctors, the nurses, the palliative care, everybody work together. All of you work together. And it’s about the patient, it’s not about getting the best part of the department or anything like that, no its about the patients” (TCIP010) |
| “the cancer it is isolating and having someone that understands, er, the journey, er, and has the compassion … I’m sure (Triggers CNS) deals with many, many patients but whenever she actually sees me or actually sees me in the corridor, er, she actually remembers me as well and she actually remembers my name…. I felt that I wasn’t being treated as like a patient, but I was treated as a person like a friend” (TCIP009) | ||
| “the more that palliative care is integrated into oncology and other hospital fields the more normalised it is… it’s about living rather than dying and I think the more integrated it is the less shock you have for the family that are going through a hard time… | ||
| Theme 8: individualising needs | “so it’s a good, er, way to navigate through how, er, cancer affects you as a person, and it’s not just from a clinical point of view, I think it’s very useful to have some things like how does it affect you spiritually, physically, mentally er, which you don’t really touch upon with your clinical team.” (TCIP009) | |
| Theme 9: perception and understanding of integrated palliative care service | “it’s not just about end of life and it’s all about managing the journey actually. I think that’s what needs to be communicated better is the management of the journey and quality of life and actually define quality of life as well” (TCIP009) | |
| “I do feel it’s changed because the words then, I can remember my mum completely panicking and all of us panicking when they said palliative care. But I don’t really panic anymore like when you say that because I see it as just an overall picture of part of the care team now.” (TSIP006) | ||
| “I would have to say the palliative care service in my mind it’s a large umbrella that, er, covers, er, the other small, the other sort of clinical things that we deal with. It’s a very good way of ensuring that you are giving yourself the best chance of survival, er, from all aspects, not just physically.” (TCIP009) |
Category 1: staff acceptability of the “Royal Marsden Triggers Tool”
Oncology staff using the “Royal Marsden Triggers Tool” felt that it was more than a palliative care referral tool because it helped reduce bias about palliative care and opened up natural discussions about palliative care (theme 1). Limitations were identified: even with a tool, limited palliative care resources, especially in the community setting, means that not every patient would be able to access palliative care services (theme 2).
Category 2: staff experience of the palliative care service based on the “Royal Marsden Triggers Tool”
For oncology staff, the use of the tool (1) normalised palliative care for both staff and patients (theme 3) and provided an opportunity to access immediate palliative care expertise in the form of communication, symptom control and practical solutions (theme 4). They acknowledged the importance of individualising care according to the needs of the patients in terms of timing and input rather than having a blanket standardised approach (theme 5) and that there may be potential organisational barriers to palliative care service delivery in terms of resources available (theme 6).
Category 3: patient experience of the palliative care service based on “Royal Marsden Triggers Tool”
Patients described how the palliative care service based on the “Royal Marsden Triggers Tool” facilitated the integration of care between the oncology and palliative care teams which normalised palliative care (theme 7). Like the staff, patients also recognised the importance of an individualised approach in terms of the timing of their introduction to palliative care and information provision and care provision (theme 8). Patients also described their experience of how being seen by the palliative care service based on the “Royal Marsden Triggers Tool” resulted in a change in their preconceived ideas from palliative care being associated with death and dying to being more about support for living well (theme 9).
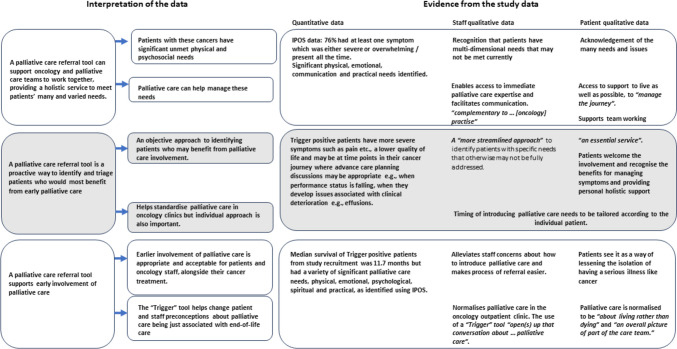
Triangulation of the quantitative and qualitative data is summarised in Fig. 1. Agreement was demonstrated between the data sources about the multidimensional needs of patients and how a palliative care referral tool enables proactive identification of patients who might benefit from access to address these needs. All data sources supported the use of such a tool to enable early palliative care involvement and reinforced the idea that palliative care is not just for end-of-life care. There were no identified areas of dissonance. There was some overlap between the emerging themes from both patients and staff which were not evaluated in the quantitative data. These synergies centred on how perception of palliative care had shifted and how the tool enabled normalisation of palliative care and supported communication and closer team working between the palliative care and oncology teams.
Fig. 1.
Triangulation of data evaluating the use, acceptability, and experience of the “Royal Marsden palliative care referral Triggers tool” in an outpatient oncology setting (direct quotations presented in italics)
Discussion
Data from this study demonstrates how the use of standardised palliative care referral criteria can underpin proactive early palliative care provision [7] and how this approach can support integration of palliative care within oncology outpatient clinics. The use of a “Trigger” tool helps triage those who would benefit most and normalises palliative care for patients who are living with, rather than dying from, cancer. The median survival of patients who were positive for the “Royal Marsden Triggers Tool” was 11 months and all were receiving anticancer treatment. Patients and staff identified that opportunities for palliative care for these outpatients were not confined to end of life care but more about proactive involvement to support them alongside cancer treatment.
The use of a palliative care referral tool supported oncology and palliative care teams to work together, providing a service that was “about the patients”. Oncology patients have significant unmet needs and early, timely, team-based palliative care can help manage these needs [5]. This was recognised by the staff and patients themselves and also recorded through the objective lens of patient-reported outcome measure, IPOS. These data demonstrate the multidimensional care needs of cancer outpatients and support the requirements for a team-based multidisciplinary approach [29] to provide holistic “umbrella” support [30] alongside oncology treatment.
In this study, we present how palliative care referral criteria may form part of a standardised care pathway to underpin the organisation and delivery of integrated working between oncology and palliative care [7] regardless of stage of cancer, prognosis or aim of treatment. Delivery of a service based on standardised referral “Triggers” breaks down some of the motivational, capability and opportunity-related barriers to providing integrated palliative care, including time, space, resource availability and access [31].
The use of palliative care referral criteria is an alternative and pragmatic approach to the traditional oncologist-driven referral to palliative care, the latter of which may be influenced by personal bias, time, resources, and experience. “Automatic” referral triggers are used to “augment” clinician-based referral rather than being used in isolation [32]. This was reflected in our staff and patient interview data in which the importance of tailoring the involvement of the palliative care team and timing of involvement according to individual patients’ needs was highlighted.
Normalisation is a key component of successful implementation of complex interventions [33]. Both patients and staff acknowledged the joined up working in the clinics supported by the “Royal Marsden Triggers Tool” and how this approach “normalised” and “opened up” the discussion about palliative care being part of the standard care offered to cancer patients. Patients described how their understanding and perceptions of palliative care changed because of their experience with the palliative care service based on the “Royal Marsden Triggers Tool”. They understood palliative care to be “about living rather than dying” and being “an overall picture of part of the care team”. Clear explanation of the role of the palliative care team was regarded as important to normalise involvement, a finding which mirrors other work in this area [34].
Using the study definition of palliative care needs, the sensitivity data of both tools suggest that this was a negative study. This is likely to be due to a methodological challenge in the design of this study. There is no validated reference standard to define patients with palliative care needs or patients who would benefit from specialist palliative care [35, 36]. In our study, we found that 91% of all study participants were experiencing at least one issue that was of at least moderate severity. Referral based on these criteria would overwhelm most specialist palliative care services and suggest that our original study definition of a patient with palliative care needs was, in retrospect, too broad. This impacted negatively on the sensitivity analysis, as originally defined. Prioritising palliative care referral for those patients with more severe needs may be more efficient, for example IPOS items scoring 3 or 4 (severe or overwhelming) as being indicative of requiring higher attention [37]. In our study, both tools identified patients with significant severe or overwhelming physical needs or lower quality of life and functioning, who would benefit from immediate intervention, for example, symptom control. An alternative approach may be to specify severity thresholds depending on the symptom itself, prioritising those for whom specialist palliative care interventions may be more effective [38].
Not all cancer patients need specialist palliative care at all stages in their illness [29]. Our data show that a palliative care referral tool is an acceptable way of identifying and triaging patients who would most benefit from early palliative care. This study was not designed to directly compare the “Royal Marsden Triggers Tool” and the “Delphi Study Criteria”, but in terms of structure and analytical metrics, our data suggest that both tools are similar. The “Royal Marsden Triggers Tool” and the “Delphi Study Criteria” include needs-based criteria relating to significant distress (physical and psychological). Both also include other criteria which are potential markers of a change or deterioration in health status, the beginning of progressive disease and/or functional decline [12]. Involvement of palliative care at times of change in cancer treatment or trajectory has been recently studied within a randomised controlled trial [39]. In our study, by incorporating these other multidimensional markers of palliative care complexity [36], both tools identified patients who may have an uncertain outcome or who are at a significant junction in their illness. These patients would benefit from palliative care input, even if they do not have immediate physical needs. The benefits of early patient-centred palliative care interventions for this group of patients include future care planning, support for communication and treatment decision-making, crisis prevention through proactive identification of problems (rather than reacting only when patients present with severe problems) [1, 2] and developing skills to cope with serious illness [40].
This study demonstrates the acceptability and usefulness of standardised criteria as an approach to targeted palliative care referrals. In clinical practice, the criteria included in a referral tool may need to be locally adapted [18]. The successful implementation and integration of any such tool will be influenced by data collection and communication processes, available resources including staffing and cultural acceptability. More evidence is needed about the best timing and mode of assessment, as well as thresholds for specialist palliative care referral. Routine symptom screening and the use of patient-reported outcome measures is being explored as the basis for targeted referrals [38, 39]. A better understanding of how to define the complexity of palliative care needs may also be beneficial [36].
A strength of this study is that a convergent mixed-methods approach was adopted with qualitative and quantitative data being used to answer different types of questions. Given the issues with the planned sensitivity analyses, the qualitative data is likely the most important aspect of the study. Triangulation enabled the contextualisation of the findings of both data sources to provide evidence to support the use of a palliative care referral tool to underpin integration of early palliative care as an acceptable and welcome part of the standard oncology service for patients undergoing cancer treatment.
Limitations of this study
This is a single timepoint, single-centre study based in an urban tertiary referral cancer centre with only English-speaking patients included in the interviews. Therefore, the findings may not be fully generalisable to other settings, patient cohorts or tumour groups. However, the proportion of “Royal Marsden Triggers Tool” or “Delphi Study Criteria” positive patients was similar to proportions of patients identified for palliative care referral using screening tools in other studies [14, 37, 41, 42]. The staff in our study appreciated the availability of immediate access to specialist palliative care expertise which is facilitated through the geographical proximity of the embedded service but acknowledged the resource and logistical implications associated with proactive identification of patients for referral including issues with space and capacity [10, 41].
Conclusion
Our data support the use of a palliative care referral tool, in association with usual oncology services, to facilitate streamlined and equitable access to timely palliative care. Additional longitudinal research is needed to evaluate the effectiveness and feasibility of this approach in the delivery of a sustainable integrated service providing patient-centred care. In the absence of evidence to support the use of one tool rather than another, referral criteria may need to be adapted and tailored according to the local patient population, healthcare environment and available healthcare resources.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contribution
Each named author has substantially contributed to the conception and design, or acquisition of data, or analysis and interpretation of data. Each author has been involved in drafting the article or revising it critically for important intellectual content, and each author has approved this final version for submission. Material preparation and data collection were performed by LK and YK. Data analysis was carried out by LK, YK, KM, JD, AMS and NP. All authors were involved in interpretation of the data. The first draft of the manuscript was written by LK and JD, and all authors commented on, contributed to and approved the final manuscript.
Funding
This work was supported by a Royal Marsden Cancer Charity grant RMCC05.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Ethical approval was granted by the London Harrow Research Ethics Committee (date 21st November 2018, REC reference 18/LO/1373).
Consent to participate
Written informed consent was obtained from all individual participants in the study.
Competing interests
The authors declare no competing interests.
Patient and public involvement
This study was co-produced with input from patients and members of the public.
The study was reviewed by over 30 members of The Royal Marsden Patient and Carer Research Review Panel three times (January and July 2017, October 2019). The Patient and Carer Research Review Panel is a rigorous PPI platform where there is extensive discussion (and transcription of those discussions for subsequent action) and shaping of design. The Triggers project was presented at the ideas stage, where PPI members shaped the project, as described by the addition of the qualitative element. The project was well received, with lengthy discussion and unanimous agreement about the aims of the project; panel members provided feedback and suggestions, which were incorporated into the design. A member of the panel (MP) was involved in the protocol development and submission for REC approval, study design and delivery, qualitative data analysis, review and interpretation of the results and drafting of the manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zimmermann C, Ryan S, Hannon B et al (2024) Team-based outpatient early palliative care: a complex cancer intervention. BMJ Support Palliat Care 14:e700–e709 [DOI] [PubMed]
- 2.Hui D, Bruera E (2015) Models of integration of oncology and palliative care. Ann Palliat Med [Internet] 4(3):89–98 [DOI] [PubMed]
- 3.Maltoni M, Scarpi E, Dall’Agata M, Zagonel V, Bertè R, Ferrari D et al (2016) Systematic versus on-demand early palliative care: results from a multicentre, randomised clinical trial. Eur J Cancer 65:61–8 [DOI] [PubMed] [Google Scholar]
- 4.Gaertner J, Siemens W, Meerpohl JJ, Antes G, Meffert C, Xander C et al (2017) Effect of specialist palliative care services on quality of life in adults with advanced incurable illness in hospital, hospice, or community settings: systematic review and meta-analysis. Bmj [Internet] 357:j2925. 10.1136/bmj.j2925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hui D, Hannon BL, Zimmermann C, Bruera E (2018) Improving patient and caregiver outcomes in oncology: team-based, timely, and targeted palliative care. CA Cancer J Clin 68(5):356–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hui D, Heung Y, Bruera E (2022) Timely palliative care: personalizing the process of referral. Cancers (Basel) 14(4):1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaasa S, Loge JH, Aapro M, Albreht T, Anderson R, Bruera E et al (2018) Integration of oncology and palliative care: a Lancet Oncology Commission. Lancet Oncol 19(11):e588-653 [DOI] [PubMed] [Google Scholar]
- 8.Ferrell BR, Temel JS, Temin S, Alesi ER, Balboni TA, Basch EM et al (2017) Integration of palliative care into standard oncology care: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol [Internet] 35(1):96–112 [DOI] [PubMed] [Google Scholar]
- 9.Jordan K, Aapro M, Kaasa S, Ripamonti CI, Scotté F, Strasser F et al (2018) European Society for Medical Oncology (ESMO) position paper on supportive and palliative care. Ann Oncol 29(1):36–43 [DOI] [PubMed] [Google Scholar]
- 10.Mathews J, Hannon B, Zimmermann C (2021) Models of integration of specialized palliative care with oncology. Curr Treat Options Oncol 22(5):44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glare PA, Semple D, Stabler SM, Saltz LB (2011) Palliative care in the outpatient oncology setting: evaluation of a practical set of referral criteria. J Oncol Pract [Internet] 7(6):366–70 [DOI] [PMC free article] [PubMed]
- 12.Hui D, Meng YC, Bruera S, Geng Y, Hutchins R, Mori M et al (2016) Referral criteria for outpatient palliative cancer care: a systematic review. Oncologist 21(7):895–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pigni A, Alfieri S, Caraceni AT, Zecca E, Fusetti V, Tallarita A et al (2022) Development of the palliative care referral system: proposal of a tool for the referral of cancer patients to specialized palliative care. BMC Palliat Care 21(1):209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paiva CE, Paiva BSR, Menezes D, Zanini LE, Ciorlia JB, Miwa MU et al (2020) Development of a screening tool to improve the referral of patients with breast and gynecological cancer to outpatient palliative care. Gynecol Oncol 158(1):153–157 [DOI] [PubMed] [Google Scholar]
- 15.Gemmell R, Yousaf N, Droney J (2020) “Triggers” for early palliative care referral in patients with cancer: a review of urgent unplanned admissions and outcomes. Support Care Cancer 28(7):3441–3449 [DOI] [PubMed] [Google Scholar]
- 16.Nadkarni Y, Kukec I, Gruber P, Jhanji S, Droney J (2022) Integrated palliative care: triggers for referral to palliative care in ICU patients. Support Care Cancer 30(3):2173–2181 [DOI] [PubMed] [Google Scholar]
- 17.RMPartners Cancer Alliance. https://rmpartners.nhs.uk. Accessed 11/07/2017
- 18.Hui D, Mori M, Watanabe SM, Caraceni A, Strasser F, Saarto T et al (2016) Referral criteria for outpatient specialty palliative cancer care: an international consensus. Lancet Oncol 17(12):e552–e559 [DOI] [PubMed] [Google Scholar]
- 19.Hui D, Anderson L, Tang M, Park M, Liu D, Bruera E (2020) Examination of referral criteria for outpatient palliative care among patients with advanced cancer. Support Care Cancer 28(1):295–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Creswell JW, C JD (2018) Research design: qualitative, quantitative, and mixed methods approaches, 5th edn. SAGE Publications Inc, Los Angeles [Google Scholar]
- 21.O’Cathain A, Murphy E, Nicholl J (2008) The quality of mixed methods studies in health services research. J Health Serv Res Policy 13(2):92–98 [DOI] [PubMed] [Google Scholar]
- 22.Murtagh FE, Ramsenthaler C, Firth A, Groeneveld EI, Lovell N, Simon ST et al (2019) A brief, patient- and proxy-reported outcome measure in advanced illness: validity, reliability and responsiveness of the Integrated Palliative care Outcome Scale (IPOS). Palliat Med 33(8):1045–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJFA, Flechtner H, Fleishman SB, de Haes JCJM, Kaasa S, Klee MC, Osoba DRD, Rofe PB, Schraub S, Sneeuw KCA, Sullivan MTF (1993) The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. JNC: J Natl Cancer Inst 85(5):365–76 [DOI] [PubMed] [Google Scholar]
- 24.van Vliet LM, Harding R, Bausewein C, Payne S, Higginson IJ, EUROIMPACT (2015) How should we manage information needs, family anxiety, depression, and breathlessness for those affected by advanced disease: development of a clinical decision support tool using a Delphi design. BMC Med 13:263. 10.1186/s12916-015-0449-6 [DOI] [PMC free article] [PubMed]
- 25.Bujang MA, Adnan TH (2016) Requirements for minimum sample size for sensitivity and specificity analysis. J Clin Diagn Res 10(10):YE01–YE06. 10.7860/JCDR/2016/18129.8744 [DOI] [PMC free article] [PubMed]
- 26.Fayers PM, A N, B K, G M, C D, B A (2001) The EORTC QLQ-C30 scoring manual (3rd edition), vol 30. EORTC, Brussels, pp 15–16 [Google Scholar]
- 27.Glaser BG, Strauss AL (1967) The discovery of grounded theory: strategies for qualitative research. New York. 10.1097/00006199-196807000-00014
- 28.O’Cathain A, Murphy E, Nicholl J (2010) Three techniques for integrating data in mixed methods studies. BMJ 341(sep17 1):c4587–c4587 [DOI] [PubMed] [Google Scholar]
- 29.Hui D, Bruera E (2016) Integrating palliative care into the trajectory of cancer care. Nat Rev Clin Oncol 13 [DOI] [PMC free article] [PubMed]
- 30.Zimmermann C, Mathews J (2022) Palliative care is the umbrella, not the rain-a metaphor to guide conversations in advanced cancer. JAMA Oncol 8(5):681–682. 10.1001/jamaoncol.2021.8210 [DOI] [PubMed]
- 31.Dunn S, Earp MA, Biondo P, Cheung WY, Kerba M, Tang PA et al (2021) Oncology clinicians’ challenges to providing palliative cancer care—a theoretical domains framework, pan-cancer system survey. Curr Oncol 28(2):1483–1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hui D, Mori M, Meng YC, Watanabe SM, Caraceni A, Strasser F et al (2018) Automatic referral to standardize palliative care access: an international Delphi survey. Support Care Cancer 26(1):175–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.May CR, Mair F, Finch T, MacFarlane A, Dowrick C, Treweek S et al (2009) Development of a theory of implementation and integration: normalization process theory. Implement Sci 4(1) [DOI] [PMC free article] [PubMed]
- 34.Zimmermann C, Swami N, Krzyzanowska M, Leighl N, Rydall A, Rodin G, Tannock I, Hannon B (2016) Perceptions of palliative care among patients with advanced cancer and their caregivers. CMAJ 188(10):E217–E227. 10.1503/cmaj.151171 [DOI] [PMC free article] [PubMed]
- 35.Müller E, Müller MJ, Boehlke C, Ramsenthaler C, Jäger H, Schäfer H et al (2022) Development of a screening tool for the need of specialist palliative care in oncologic inpatients: study protocol for the ScreeningPALL Study. BMJ Open 12(9):e059598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tuca A, Viladot M, Carrera G, Llavata L, Barrera C, Chicote M et al (2024) Evolution of complexity of palliative care needs and patient profiles according to the PALCOM scale (part two): pooled analysis of the cohorts for the development and validation of the PALCOM scale in advanced cancer patients. Cancers (Basel) 16(9) [DOI] [PMC free article] [PubMed]
- 37.Ostgathe C, Wendt KN, Heckel M, Kurkowski S, Klein C, Krause SW, Fuchs FS, Bayer CM, Stiel S (2019) Identifying the need for specialized palliative care in adult cancer patients - development and validation of a screening procedure based on proxy assessment by physicians and filter questions. BMC Cancer 19(1):646. 10.1186/s12885-019-5809-8 [DOI] [PMC free article] [PubMed]
- 38.Zimmermann C, Pope A, Hannon B, Bedard PL, Rodin G, Dhani N et al (2023) Symptom screening with Targeted Early Palliative care (STEP) versus usual care for patients with advanced cancer: a mixed methods study. Support Care Cancer 31(7) [DOI] [PubMed]
- 39.Temel JS, Jackson VA, El-Jawahri A, Rinaldi SP, Petrillo LA, Kumar P et al (2024) Stepped palliative care for patients with advanced lung cancer: a randomized clinical trial. JAMA 332(6):471–481. 10.1001/jama.2024.10398 [DOI] [PMC free article] [PubMed]
- 40.Greer JA, Pirl WF, Jackson VA, Muzikansky A, Lennes IT, Heist RS et al (2012) Effect of early palliative care on chemotherapy use and end-of-life care in patients with metastatic non–small-cell lung cancer. J Clin Oncol 30(4):394–400 [DOI] [PubMed] [Google Scholar]
- 41.Molin Y, Gallay C, Gautier J, Lardy-Cleaud A, Mayet R, Grach MC et al (2019) PALLIA-10, a screening tool to identify patients needing palliative care referral in comprehensive cancer centers: a prospective multicentric study (PREPA-10). Cancer Med 8(6):2950–2961. 10.1002/cam4.2118 [DOI] [PMC free article] [PubMed]
- 42.Le QV, Trinh HL, Mai KNT, Pham MD, Glare PA (2020) Screening patients with cancer admitted to Hanoi Medical University Hospital for palliative care needs. JCO Glob Oncol 6:1321–1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.