Abstract
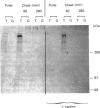
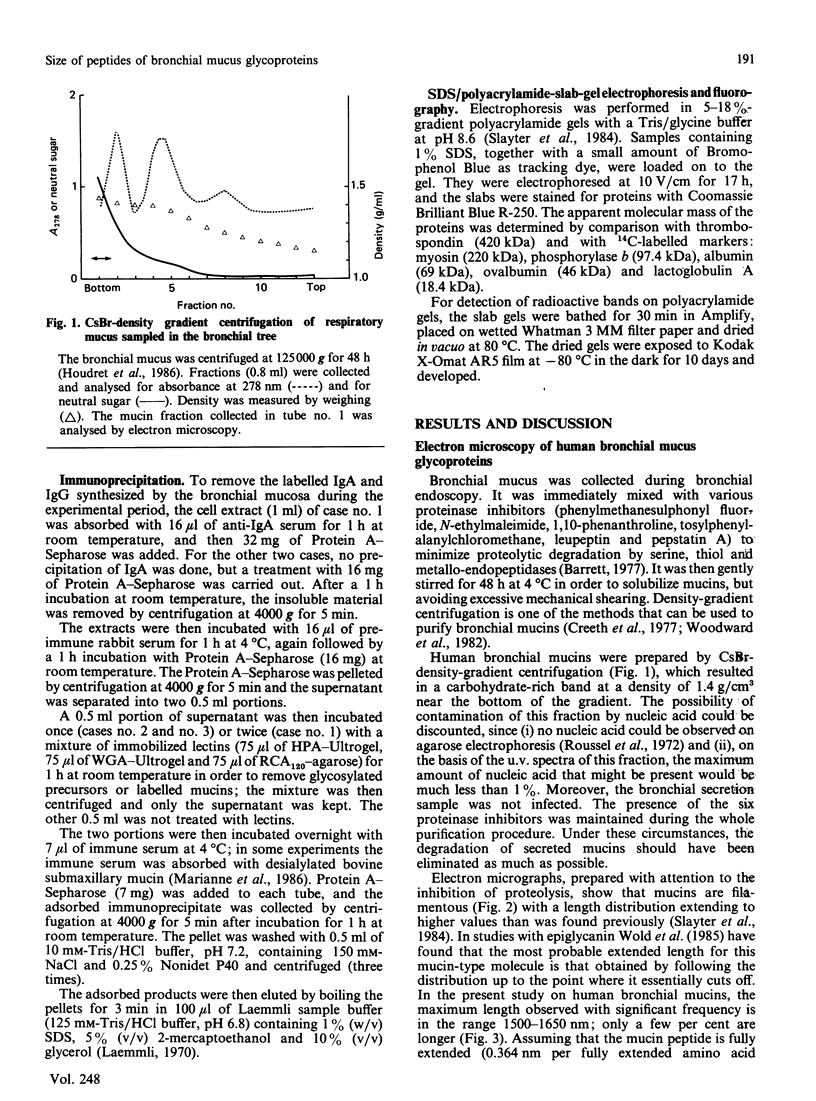
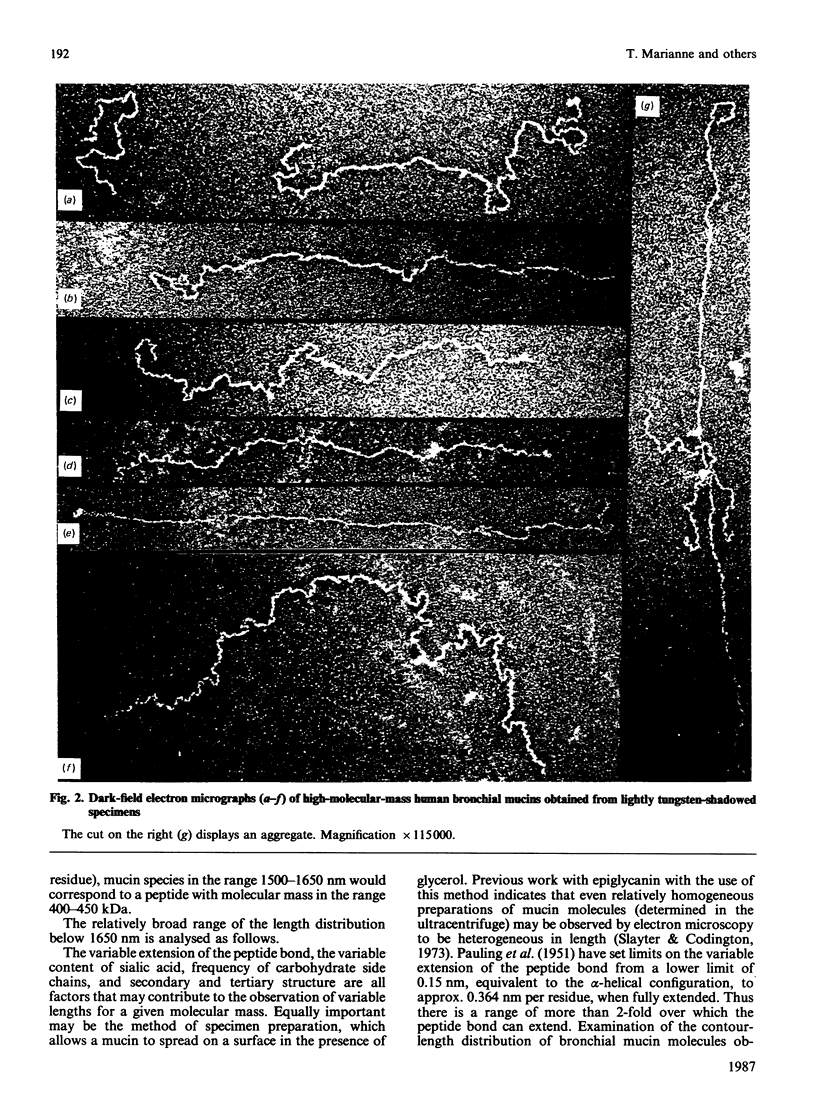
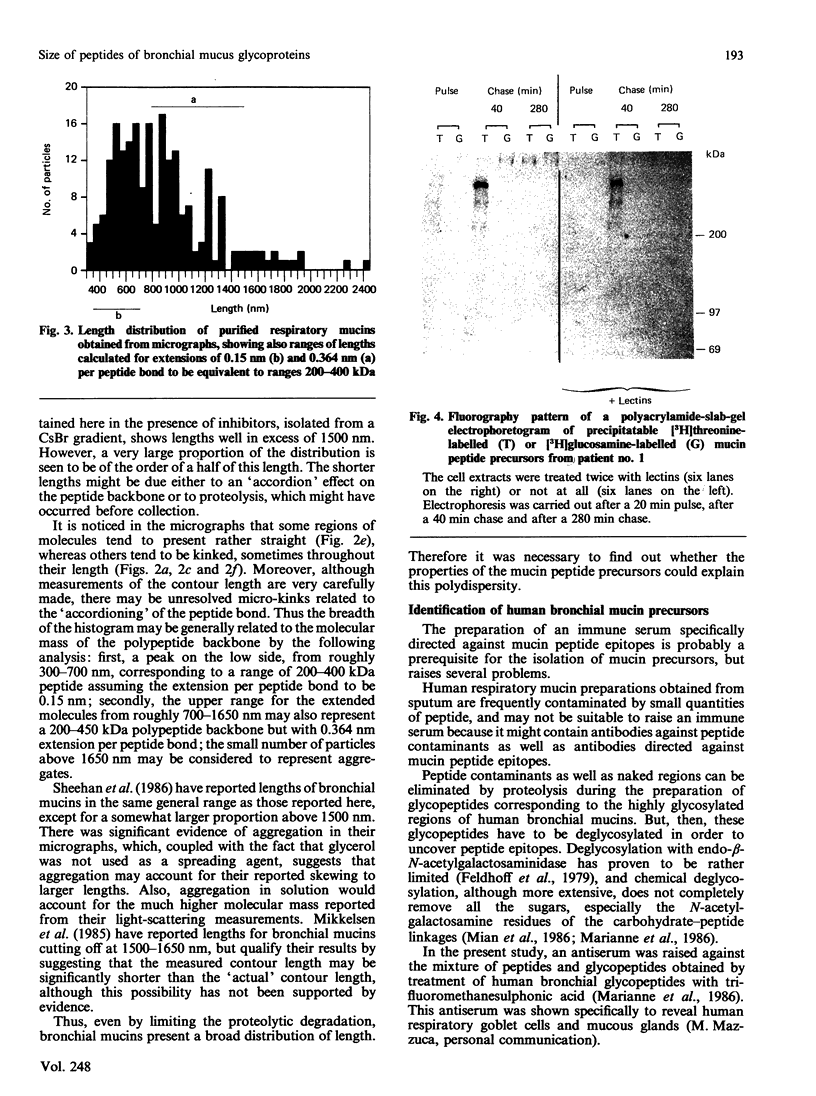
Secreted human bronchial mucins, directly collected from macroscopically healthy bronchial mucosa, were prepared in the presence of six proteinase inhibitors, and analysed by electron microscopy. These mucins were similar in length distribution to molecules prepared from sputum [Slayter, Lamblin, Le Treut, Galabert, Houdret, Degand & Roussel (1984) Eur. J. Biochem. 142, 209-218], although they were a little longer, their lengths ranging up to about 1,650 nm. This length corresponds to an extended mucin peptide of about 450 kDa. In order to compare these peptide lengths with the molecular size of biosynthetic precursors, an antiserum raised against trifluoromethanesulphonic acid-treated highly glycosylated regions of human bronchial mucins was used to isolate mucin precursors synthesized in explants of human bronchial mucosa during pulse-labelling with [3H]threonine or [3H]glucosamine. A main precursor labelled with [3H]threonine and with an apparent molecular mass of about 400 kDa was detected by fluorography following SDS/polyacrylamide-gel electrophoresis. This band was observed as early as 20 min; it was more intense after a 40 min chase and had disappeared after a chase period of 280 min in unlabelled medium, presumably owing to glycosylation. Much fainter bands at about 200 kDa and between 200 and 400 kDa, also labelled with [3H]threonine, were observed mainly after a 40 min chase and had disappeared after a 280 min chase. None of these bands was labelled with [3H]glucosamine, nor did they disappear after multiple treatments with immobilized lectins. After a 280 min chase, [3H]threonine-labelled material appeared in the stacking gel, which also contained [3H]glucosamine label. The results indicate that the 200-400 kDa species are mucin precursors, whose size is comparable with that obtained by electron microscopy for respiratory mucins collected directly from the macroscopically healthy bronchial mucosa.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carlstedt I., Sheehan J. K., Corfield A. P., Gallagher J. T. Mucous glycoproteins: a gel of a problem. Essays Biochem. 1985;20:40–76. [PubMed] [Google Scholar]
- Creeth J. M., Bhaskar K. R., Horton J. R., Das I., Lopez-Vidriero M. T., Reid L. The separation and characterization of bronchial glycoproteins by density-gradient methods. Biochem J. 1977 Dec 1;167(3):557–569. doi: 10.1042/bj1670557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldhoff P. A., Bhavanandan V. P., Davidson E. A. Purification, properties, and analysis of human asthmatic bronchial mucin. Biochemistry. 1979 May 29;18(11):2430–2436. doi: 10.1021/bi00578a044. [DOI] [PubMed] [Google Scholar]
- Houdret N., Perini J. M., Galabert C., Scharfman A., Humbert P., Lamblin G., Roussel P. The high lipid content of respiratory mucins in cystic fibrosis is related to infection. Biochim Biophys Acta. 1986 Jan 15;880(1):54–61. doi: 10.1016/0304-4165(86)90119-4. [DOI] [PubMed] [Google Scholar]
- Jenssen A. O., Harbitz O., Smidsrød O. Electron microscopy of mucin from sputum in chronic obstructive bronchitis. Eur J Respir Dis. 1980 Apr;61(2):71–76. [PubMed] [Google Scholar]
- Laboisse C. L. Structure of gastrointestinal mucins: searching for the Rosetta stone. Biochimie. 1986 May;68(5):611–617. doi: 10.1016/s0300-9084(86)80155-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marianne T., Perini J. M., Houvenaghel M. C., Tramu G., Lamblin G., Roussel P. Action of trifluoromethanesulfonic acid on highly glycosylated regions of human bronchial mucins. Carbohydr Res. 1986 Aug 15;151:7–19. doi: 10.1016/s0008-6215(00)90325-2. [DOI] [PubMed] [Google Scholar]
- Mazzuca M., Lhermitte M., Lafitte J. J., Roussel P. Use of lectins for detection of glycoconjugates in the glandular cells of the human bronchial mucosa. J Histochem Cytochem. 1982 Sep;30(9):956–966. doi: 10.1177/30.9.7130674. [DOI] [PubMed] [Google Scholar]
- Mikkelsen A., Stokke B. T., Christensen B. E., Elgsaeter A. Flexibility and length of human bronchial mucin studied using low-shear viscometry, birefringence relaxation analysis, and electron microscopy. Biopolymers. 1985 Sep;24(9):1683–1704. doi: 10.1002/bip.360240904. [DOI] [PubMed] [Google Scholar]
- PAULING L., COREY R. B., BRANSON H. R. The structure of proteins; two hydrogen-bonded helical configurations of the polypeptide chain. Proc Natl Acad Sci U S A. 1951 Apr;37(4):205–211. doi: 10.1073/pnas.37.4.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts G. P. The role of disulfide bonds in maintaining the gel structure of bronchial mucus. Arch Biochem Biophys. 1976 Apr;173(2):528–537. doi: 10.1016/0003-9861(76)90289-7. [DOI] [PubMed] [Google Scholar]
- Rose M. C., Voter W. A., Sage H., Brown C. F., Kaufman B. Effects of deglycosylation on the architecture of ovine submaxillary mucin glycoprotein. J Biol Chem. 1984 Mar 10;259(5):3167–3172. [PubMed] [Google Scholar]
- Roussel P., Lamblin G., Degand P., Havez R. Isolement des mucines bronchiques secretées au cours de la mucoviscidose. Clin Chim Acta. 1972 Feb;36(2):315–328. doi: 10.1016/0009-8981(72)90005-8. [DOI] [PubMed] [Google Scholar]
- Sheehan J. K., Oates K., Carlstedt I. Electron microscopy of cervical, gastric and bronchial mucus glycoproteins. Biochem J. 1986 Oct 1;239(1):147–153. doi: 10.1042/bj2390147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slayter H. S., Codington J. F. Size and configuration of glycoprotein fragments cleaved from tumor cells by proteolysis. J Biol Chem. 1973 May 25;248(10):3405–3410. [PubMed] [Google Scholar]
- Slayter H. S. High-resolution metal replication of macromolecules. Ultramicroscopy. 1976 Sep-Oct;1(4):341–357. doi: 10.1016/0304-3991(76)90050-4. [DOI] [PubMed] [Google Scholar]
- Slayter H. S., Lamblin G., Le Treut A., Galabert C., Houdret N., Degand P., Roussel P. Complex structure of human bronchial mucus glycoprotein. Eur J Biochem. 1984 Jul 16;142(2):209–218. doi: 10.1111/j.1432-1033.1984.tb08273.x. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Stafford W. F., 3rd, Slayter H. S., Seidel J. C. A conformational transition in gizzard heavy meromyosin involving the head-tail junction, resulting in changes in sedimentation coefficient, ATPase activity, and orientation of heads. J Biol Chem. 1985 Nov 25;260(27):14810–14817. [PubMed] [Google Scholar]
- Trump B. F., Resau J., Barrett L. A. Methods of organ culture for human bronchus. Methods Cell Biol. 1980;21A:1–14. doi: 10.1016/s0091-679x(08)60755-4. [DOI] [PubMed] [Google Scholar]
- Wold J. K., Slayter H. S., Codington J. F., Jeanloz R. W. Location of an epitopic site on epiglycanin by molecular immunoelectron microscopy. Biochem J. 1985 Apr 1;227(1):231–237. doi: 10.1042/bj2270231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward H., Horsey B., Bhavanandan V. P., Davidson E. A. Isolation, purification, and properties of respiratory mucus glycoproteins. Biochemistry. 1982 Feb 16;21(4):694–701. doi: 10.1021/bi00533a017. [DOI] [PubMed] [Google Scholar]