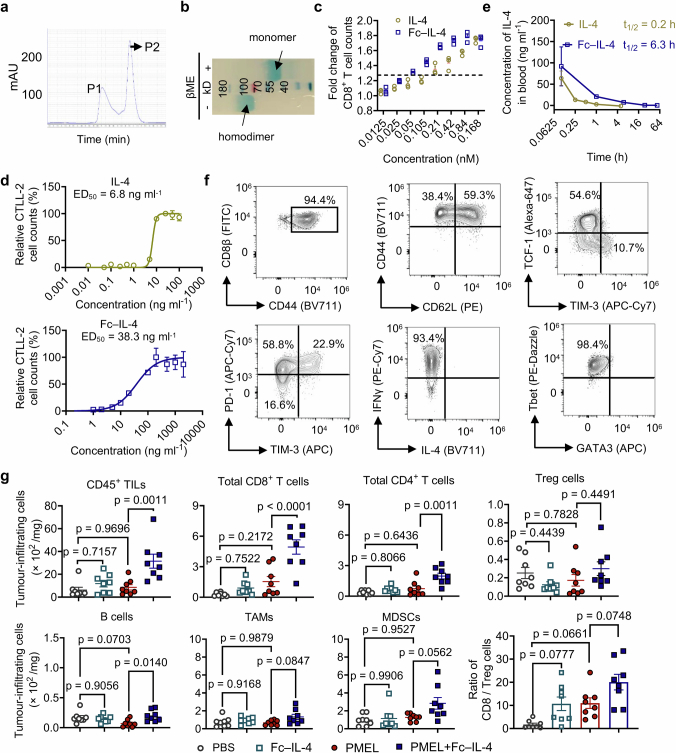
Extended Data Fig. 1. Characterizations of Fc–IL-4 and investigation of its impact on tumour-infiltrating immune cells.
a, Representative size-exclusion chromatographic traces of Fc–IL-4 fusion protein. Peak 2 (P2) was collected and analysed. b, SDS-PAGE analysis of purified Fc–IL-4. βME, β-mercaptoethanol (Image is one representative of two independent experiments). c, Fold change of PMEL T cell counts (normalized by that in PBS group) upon treatment of IL-4 or Fc–IL-4 at equivalent concentrations in vitro. d, Median effective dose (ED50) of native IL-4 and Fc–IL-4 was determined using the CTLL-2 proliferation assay. e, The pharmacokinetics in plasma and half-life of IL-4 and Fc–IL-4 (n = 3 animals). f, The phenotype, cytokine production, and expression of transcription factors of activated PMEL T cells prior to transfer. g, Experimental setting was described in Fig. 1a. Shown are counts of various tumour-infiltrating immune cells. Treg, regulatory CD4+ T cells; TAMs, tumour-associated macrophages; MDSCs, myeloid-derived suppressive cells. Data are one representative of three independent experiments with n = 3 biological replicates (c, and d) or one representative of two independent experiments with n = 8 animals (g). All data represent mean ± s.e.m. and are analysed by one-way ANOVA and Tukey’s test.