Abstract
BACKGROUND
The number of people with dementia is increasing in Japan, and establishing evidence for preventing dementia is necessary.
METHODS
This study was a randomized controlled trial in cognitively normal community‐dwelling older adults aged 65 to 85 with diabetes and/or hypertension. Participants were randomly assigned in a 1:1 ratio. The intervention group underwent 90 min of group‐based weekly physical exercise, cognitive training, nutritional counseling, and vascular risk management for 18 months. The primary endpoint was the change in a cognitive composite score calculated by averaging the z‐scores of seven neuropsychological tests from baseline to 18 months.
RESULTS
We randomly assigned 203 participants to two groups, and 178 (87.7%) completed the 18‐month follow‐up. There was a significant group difference in the cognitive composite score change at 18 months (mean difference 0.16, 95% confidence interval: 0.04 to 0.27; p = 0.009).
DISCUSSION
An 18‐month multimodal intervention for older adults at risk of dementia could improve their cognitive function. The trial was registered in the Clinical Trial Registration System (UMIN000041938).
Highlights
Japan‐Multimodal Intervention Trial for Prevention of Dementia (J‐MINT) PRIME Tamba was a randomized controlled trial to prevent dementia.
We provided a multifactorial intervention based on the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER) trial methodology.
The primary outcome, the cognitive composite score, improved with our intervention.
Executive function/processing speed and memory improved in the intervention group.
Intervention adherence was high, and no serious adverse events occurred.
Keywords: cognitive function, dementia, J‐MINT PRIME Tamba, multimodal intervention, prevention, randomized controlled trial, World‐Wide FINGERS
1. BACKGROUND
The number of people living with dementia worldwide in 2019 has been estimated at 55.2 million and is expected to grow to 139 million by 2050. 1 Japan has the highest aging rate in the world, and it is estimated that one in five older adults will have dementia by 2025, 2 making it a social problem. Recently, the Japanese government approved a disease‐modifying therapy called lecanemab, 3 , 4 but it cannot prevent the deterioration of cognitive functions in patients with Alzheimer's disease once the symptoms appear. From this viewpoint, prevention strategies are essential, and non‐pharmacological interventions are also receiving attention.
Risk factors for cognitive impairment and dementia vary by age, and potentially modifiable lifestyle risk factors include smoking, physical inactivity, diabetes, depression, social isolation, and cognitive inactivity. 5 A 2‐year multifactorial intervention (the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability [FINGER]) targeting these risk factors showed improved cognitive function and was an effective preventive strategy for older adults at risk of dementia. 6
Based on these results, studies have been conducted worldwide to test the effectiveness of multidomain interventions. In 2017, the World‐Wide FINGERS (WW‐FINGERS) network was launched based on the FINGER methodology. Over 60 countries currently participate in this network, which aims to accumulate data and generalize strategies to prevent cognitive impairment and dementia by promoting new clinical research practices. 7 Similar initiatives inside and outside WW‐FINGERS have also been successfully implemented, particularly in Asia. 8 , 9 In Japan, the Japan‐Multimodal Intervention Trial for Prevention of Dementia (J‐MINT) 10 was started in 2019 and was included in the WW‐FINGERS network. The J‐MINT PRIME Tamba study is a randomized controlled trial (RCT) that uses a protocol similar to that of the J‐MINT study and is an extension study conducted in the city of Tamba by Kobe University. Tamba is in the northeastern region of Hyogo Prefecture, Japan, with approximately 62,000 residents and the aging rate for those 65 and over is 35% as of April 2022. This region is characterized by a substantial older population and a high rate of people at risk of vascular diseases, such as hypertension and diabetes.
We designed a RCT to examine the efficacy of a multidomain dementia prevention program consisting of lifestyle‐related disease management, physical exercise, nutritional counseling, and cognitive training to improve or maintain cognitive function or reduce cognitive decline in older adults at risk of dementia.
2. METHODS
The study protocol is described in detail in a previously published paper 11 and the Supplemental Materials (pp. 2 to 22) of this article.
2.1. Study design
The J‐MINT PRIME Tamba trial was designed as a RCT of community‐dwelling older adults in Tamba, Hyogo, Japan, with a 1:1 allocation ratio between the intervention and control groups. Baseline assessments of both groups were conducted in September 2020, followed by assessments every 6 months. The intervention group received 78 weekly interventions over 18 months beginning in October 2020.
2.2. Participants
The inclusion criteria were as follows: residents of Tamba, aged 65 to 85 years at enrollment, Dementia Assessment Sheet in the Community‐based Care System‐21 items (DASC‐21) 12 score from 22 to 30, and those with at least one of the following vascular risks: under treatment for hypertension; systolic blood pressure ≥140 mmHg; diastolic blood pressure ≥85 mmHg; under treatment for diabetes; hemoglobin A1c (HbA1c) ≥ 6.0%. Those with a Mini‐Mental State Examination (MMSE) 13 score <24 and those already receiving public long‐term care were excluded from the study.
To recruit participants, those who had undergone the DASC‐21 among those who had undergone special health check‐ups conducted by Tamba City between April 2019 and March 2020 for those aged 65 years and older were invited by mail to participate in the study. Additional participants were recruited using newspaper inserts and local press releases. After being briefed on the study content, the participants were screened for DASC‐21, MMSE, and vascular risk status. Those who met the eligibility criteria were given detailed explanations orally and in writing and were enrolled in the study after signing a consent form.
The J‐MINT PRIME Tamba research plan was reviewed and approved by the Kobe University Ethics Committee for Health Sciences (Approval No.: 922). The trial was registered in the University Hospital Medical Information Network Clinical Trial Registration System (UMIN000041938). All participants were fully informed about the study, understood the benefits and potential risks of participation, and provided written informed consent.
2.3. Randomization and masking
Based on age, sex, and MMSE score information obtained at screening, participants were randomized into one of two groups at a 1:1 ratio using a dynamic allocation method with stratification factors. The stratification criteria were as follows: (1) age, 65 to 74 or 75 to 85 years; (2) sex, female or male; and (3) MMSE score, 24 to 27 or 28 to 30. The researchers enrolled participants in an electronic data capture (EDC) system, and an external organization performed dynamic allocation using an algorithm blinded to the researchers and participants.
All participants knew their allocation group, but the assessors and intervention advocates were blinded to the allocation and were not involved in the intervention activities at each time point. For the researchers and administrators with access to the EDC system, one researcher and three administrators were present at the intervention site for their risk management and reception roles during physical exercise. However, they were not involved in collaborative intervention or assessment at each timepoint.
2.4. Procedures
All participants underwent physical function assessment and neuropsychological testing by trained physical therapists, occupational therapists, and nurses at baseline, 6, 12, and 18 months. Blood samples were collected at baseline, 6 months, and 18 months. To avoid compromising the effectiveness of the intervention, the results of each 6‐month assessment were not disclosed to participants until the end of the study, except in the case of serious findings.
The intervention group received multidomain interventions, including lifestyle‐related disease management, physical exercise, nutritional counseling, and cognitive training. First, lifestyle‐related diseases were managed. According to clinical practice guidelines, 14 , 15 , 16 participants with diabetes, hypertension, or dyslipidemia were managed by supervisors who were health professionals, such as public health nurses and nutritionists.
Then came the physical exercise. Participants underwent an exercise program consisting of 50‐min aerobic exercise, 20‐min dual‐task exercise, resistance training, and 20‐min group meetings for a total of 90 min per session once a week for 18 months. The participants’ pulse rate determined the intensity of the aerobic exercise, which gradually increased from 40% to 80%. Program instructors were qualified health professionals, such as physical and occupational therapists. Nutrition counseling followed. Participants received dietary counseling from health professionals, such as public health nurses, nurses, and dieticians, through face‐to‐face interviews at 1, 7, and 13 months and telephone follow‐ups every 5 weeks after the visits. The dietary advice program consisted of dietary assessment; behavioral goal setting; consumption of foods suitable for dementia prevention such as fish, chicken, beans/soy products, vegetables/seaweed, seasonal foods, and colorful and varied food combinations; and oral care advice on oral frailty. Finally, for cognitive training, participants underwent cognitive training using a tablet with the BrainHQ software program (Posit Science, San Francisco, CA, USA). Nestle Japan (Tokyo, Japan) operated the version of BrainHQ used in this study, which consisted of 13 visual exercises focusing on specific cognitive abilities, such as attention, processing speed, memory, mental flexibility, and visuospatial ability. Exercise difficulty was adjusted based on the cognitive abilities of each individual to ensure and sustain engagement of attention and motivation. Participants underwent cognitive function training for at least 30 min per day, ≥4 days/week, with practice and rest periods every 3 months. Every 3 months, they received feedback on changes in their performance over time.
RESEARCH IN CONTEXT
Systematic review: The authors reviewed the literature on multimodal interventions for the prevention of dementia. Published systematic reviews have found slight improvements in cognitive function in groups of participants who received multimodal interventions. However, there is no clear evidence and no specific views on intervention methods or assessment items.
Interpretation: This study is the first well‐designed, randomized controlled trial of a multifactorial intervention for older adults in Japan. Our results showed improvements in global cognitive composite score and physical function. Despite the relatively short intervention period of 18 months, the mean difference in global cognitive composite scores between groups in this study was larger than in previous studies.
Future directions: Multimodal interventions can improve and maintain cognitive and physical function in older populations. The important question that arises is how to promote social implementation so that many older people can benefit from the results of this research.
The control group received written health‐related information every 2 months on how to prevent/manage dementia, frailty, low back pain, malnutrition, health‐related illnesses, sleep disorders, falls, and fall‐related fractures; the benefits of social participation and physical activity; and the management of vascular risk factors according to current guidelines.
2.5. Outcomes
The primary outcome measure was the change from baseline at 18 months in a global cognitive composite score using several neuropsychological tests, including tests of global cognitive function (MMSE); memory (Logical Memory I and II subset of the Wechsler Memory Scale‐Revised [WMS‐R] 17 and Free and Cued Selective Reminding Test [FCSRT] 18 ); and attention (Digit Span of the Wechsler Adult Intelligence Scale [WAIS‐III] 19 ), executive function/processing speed (Trail‐Making Test [TMT], 20 Digit Symbol Substitution Test (DSST) subset of the WAIS‐III, and Letter Word Fluency Test 20 ). The neuropsychological test scores for each participant in each period were standardized using the baseline assessment's mean and standard deviation (SD) from the full population analysis set.
Secondary outcome measures included changes in global composite scores from baseline to 6 and 12 months for cognitive function; changes from baseline to 6, 12, and 18 months for each neuropsychological test; changes from baseline to 6, 12, and 18 months for blood test values; changes in activities of daily living (ADL) 21 , 22 function and frailty 23 , 24 , 25 from baseline to 6, 12, and 18 months; and changes in digital cognitive tests (Cogstate Brief Battery; Cogstate, Melbourne, Australia) 26 from baseline to 18 months.
Post hoc analyses were performed to measure changes in each domain (memory, attention, and executive function/processing speed) cognitive composite score from baseline to 18 months and in walking speed, grip strength, five times sit‐to‐stand test (FTSST), and inspiratory and expiratory muscle strength, measured as maximal inspiratory pressure (PImax) and maximal expiratory pressure (PEmax) from baseline to 18 months.
Information on adherence, such as the participation rate in face‐to‐face interventions and cognitive training at home, was recorded. All adverse events were recorded, and all participants were asked at the 6‐, 12‐, and 18‐month visits whether they had experienced any study‐related adverse events such as falls or musculoskeletal pain.
2.6. Statistical analyses
The sample size was estimated from a meta‐analysis of RCTs using cognitive training as an intervention. According to this meta‐analysis, overall cognitive function improved in the intervention group, with Hedges’ g of 0.419. 27 The sample size required for a two‐tailed t test with a 5% risk rate, 80% power, and a 1:1 allocation ratio was 182 participants, assuming a similar effect size in the present study. We assumed a dropout rate of approximately 10% from baseline to 18 months and set a minimum sample size of 200 participants. To calculate the composite score for cognitive function, we first calculated the z‐scores for the MMSE, Logical Memory I and II, FCSRT, Digit Span, TMT, DSST, and Letter Word Fluency Test using the baseline mean and SD. The composite score was the mean z‐score for each assessment item (included in Supplemental materials, Figure S1). If some values were missing, composite scores were calculated using only items that could be measured. The sign was reversed for the TMT only because the lower the value, the better the cognitive function. A mixed model for repeated measures (MMRM) was used for the primary endpoint, the cognitive function composite score changes from baseline to 18 months between the intervention and control groups. The change in the composite score at each time point was used as the objective variable, and the randomization group, evaluation time point, interaction between the randomization group and evaluation time point, age group at allocation (<75 years, ≥75 years), sex, and baseline cognitive composite score were used as fixed effects. The covariance structure was assumed to be unstructured, and the degrees of freedom were adjusted using Kenward–Roger approximation. 28 The Bonferroni adjustment method was used for between‐group comparisons.
For the secondary outcomes of physical, social, and oral frailty, proportions and 95% confidence intervals (CIs) were calculated for each group at each time point, with the 95% CIs being calculated using the Clopper–Pearson method. 29 Cases and proportions at each time point were calculated and compared using generalized estimating equations (GEEs) with logit link functions. Effects and covariates included in the model were the same as for the primary outcome. The type of correlation coefficient matrix was unstructured. The incidences of serious adverse events were pooled between the intervention and control groups. The frequency and incidence of adverse events were also tabulated, and 95% CIs were calculated using the Clopper–Pearson method. For demographic characteristics and other items, means and SDs for continuous variables and proportions for categorical variables were used as indices. A significance level of less than 5% was used for all analyses. SPSS Statistics 29 (IBM, Armonk, NY, USA) was used for all calculations, and GraphPad Prism 9 (GraphPad Software, San Diego, CA, USA) was used to generate the figures.
3. RESULTS
Between September 16, 2020, and October 15, 2020, 206 individuals were screened and 203 were randomized to the intervention (n = 101) or control group (n = 102; Figure 1). A total of 189 (93.1%), 185 (91.1%), and 178 (87.7%) participants completed the 6‐, 12‐, and 18‐month assessments, respectively. Three participants refused the neuropsychological tests at the final assessment, so they received only other assessments. There was no significant difference in dropout rates between the intervention (eight participants [7.9%]) and control groups (17 participants [16.7%]). The main reasons for dropping out were family‐related issues (n = 11 [44.0%]), health reasons (n = 5 [20.8%]), and lack of motivation for the study (n = 4 [16.7%]). Four participants died during the study. The baseline characteristics of the intervention and control groups were similar (Table 1). The mean (SD) age, years of education, and MMSE and DASC‐21 scores of the population were 73.9 (4.8), 12.6 (1.9), 28.7 (1.4), and 24.1 (1.9), respectively. The proportion of apolipoprotein E epsilon 4 carriers was 18.7% (Table S1). The study participants in both groups had a daily exercise routine, very few smokers, and a very high fish intake, which means they had a high level of health consciousness.
FIGURE 1.
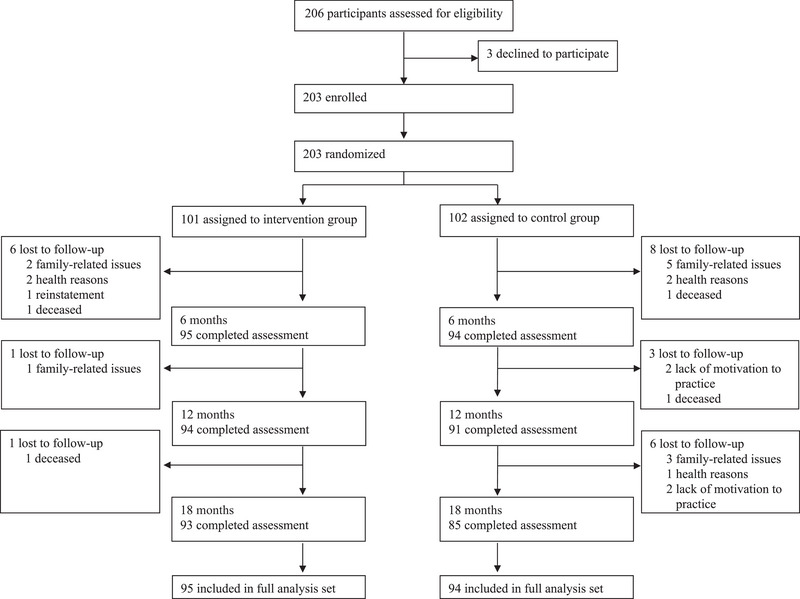
Trial profile: flow diagram showing process of enrollment, allocation, follow‐up, and analysis for the J‐MINT PRIME Tamba. J‐MINT, Japan‐Multimodal Intervention Trial for Prevention of Dementia.
TABLE 1.
Baseline characteristics of participants.
| Characteristics at baseline | Participants with information available | Intervention group (n = 101) | Control group (n = 102) |
|---|---|---|---|
| Demographic characteristics | |||
| Age at baseline visit (years) | 203 | 74.28 (4.76) | 73.52 (4.88) |
| Sex (female, %) | 203 | 75 (74.3) | 70 (68.6) |
| Education (years) | 203 | 12.53 (1.99) | 12.72 (1.80) |
| Vascular factors | |||
| Systolic blood pressure (mmHg) | 203 | 142.41 (16.94) | 146.89 (15.66) |
| Diastolic blood pressure (mmHg) | 203 | 81.66 (9.93) | 82.70 (10.77) |
| Serum total cholesterol (mg/ml) | 203 | 207.8 (32.69) | 202.72 (32.83) |
| Hemoglobin A1c (%) | 203 | 5.71 (0.68) | 5.80 (0.55) |
| Body mass index (kg/m2) | 203 | 22.78 (3.48) | 23.37 (3.29) |
| Lifestyle‐related factors | |||
| Physical activity at least once/week (%) | 201 | 82 (83.7) | 75 (73.5) |
| Current smokers (%) | 201 | 1 (1.0) | 3 (3.0) |
| Alcohol drinking at least once/week (%) | 201 | 36 (36.3) | 41 (40.1) |
| Fish intake at least twice/week (%) | 201 | 100 (100) | 101 (99.0) |
| Daily intake of vegetables (%) | 201 | 70 (70.7) | 65 (63.7) |
| Self‐reported medical conditions | |||
| Hypertension (%) | 203 | 58 (57.4) | 63 (61.7) |
| Hypercholesterolemia (%) | 203 | 38 (37.6) | 27 (26.5) |
| Diabetes (%) | 203 | 23 (22.8) | 24 (23.5) |
| History of stroke (%) | 203 | 9 (8.9) | 0 (0) |
| Cancer (%) | 203 | 11 (10.9) | 12 (11.8) |
| Atrial fibrillation (%) | 203 | 4 (4.0) | 3 (3.0) |
| Congestive heart failure (%) | 203 | 1 (1.0) | 0 (0) |
| Chronic kidney failure (%) | 203 | 5 (5.0) | 1 (1.0) |
| Liver disease (%) | 203 | 2 (2.0) | 5 (5.0) |
| Coronary artery disease (%) | 203 | 3 (3.0) | 6 (6.0) |
| Parkinson's disease (%) | 203 | 0 (0) | 0 (0) |
| Depression (%) | 203 | 2 (2.0) | 3 (3.0) |
| Insomnia (%) | 203 | 3 (3.0) | 8 (8.0) |
| Asthma (%) | 203 | 5 (5.0) | 5 (5.0) |
| Chronic obstructive pulmonary disease (%) | 203 | 1 (1.0) | 0 (0) |
| Interstitial lung disease (%) | 203 | 0 (0) | 0 (0) |
| Cognition | |||
| Global composite score | 203 | −0.02 (0.58) | 0.01 (0.57) |
| Attention | 201 | 0.01 (0.81) | −0.01 (0.88) |
| Executive functioning/processing speed | 201 | −0.01 (0.75) | 0.01 (0.70) |
| Memory | 201 | −0.02 (0.86) | 0.01 (0.72) |
| Mini‐Mental State Examination | 203 | 28.70 (1.41) | 28.60 (1.31) |
| DASC‐21 | 203 | 24.11 (1.98) | 24.12 (1.89) |
| Physical function | |||
| Gait speed (m/s) | 201 | 1.30 (0.23) | 1.30 (0.25) |
| Grip strength of male (kg) | 57 | 35.75 (6.85) | 37.09 (5.63) |
| Grip strength of female (kg) | 144 | 24.20 (3.58) | 24.20 (2.87) |
| FTSST (s) | 201 | 9.57 (2.91) | 9.50 (2.72) |
| Inspiratory muscle strength | 201 | 51.69 (19.74) | 52.13 (27.85) |
| Expiratory muscle strength | 201 | 67.97 (22.42) | 69.87 (21.79) |
| Frailty status | |||
| Physical frailty | 201 | 4 (4.0) | 1 (1.0) |
| Social frailty | 200 | 28 (28.6) | 34 (33.3) |
| Oral frailty | 200 | 41 (41.8) | 30 (29.4) |
Notes: Data are presented as n, n (%), or mean (standard deviation). The analysis was performed in a modified intention‐to‐treat population (participants underwent at least one post‐baseline evaluation of the primary efficacy endpoint).
Scores on the global composite score, attention, executive functioning/processing speed, and memory are the mean values of the z‐scores of the cognitive tests included in each cognitive outcome, with higher scores indicating better performance.
Abbreviations: DASC‐21, 21‐item Dementia Assessment Sheet for the Community‐based Integrated Care System; FTSST, five times sit‐to‐stand test.
The intervention had a significant beneficial effect on the global cognitive composite score (Figure 2, Table 2, and Tables S2 and S3). The estimated mean change in the global composite score at 18 months was 0.55 (standard error [SE] 0.04) in the intervention group and 0.39 (SE 0.04) in the control group, indicating a statistically significant group difference (mean difference 0.16, 95% CI 0.04 to 0.27; p = 0.009). At 18 months, the improvement in the global composite score was 41.0% higher in the intervention group than in the control group. There were also significant intervention effects on the post hoc analysis results for executive function/processing speed (p = 0.03) and memory function (p = 0.04; Figure 2, Table 2). However, there was no significant difference in attention between the groups.
FIGURE 2.
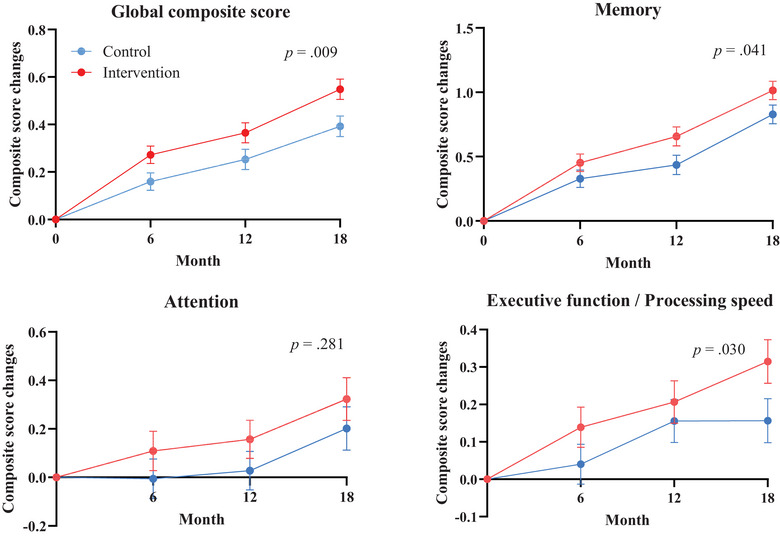
Change in cognitive performance during 18‐month intervention. The figure shows the estimated mean change in cognitive performance from baseline to 6, 12, and 18 months (higher scores suggest better performance) in the full analysis set. Error bars are standard errors. Mixed models for repeated measures analyses were used to assess between‐group differences in changes from baseline to 18 months based on participant data with at least one post‐baseline measurement. Global composite scores were calculated using all neuropsychological tests. Memory‐domain composite scores were calculated using the Logical Memory of the Wechsler Memory Scale‐Revised and the Free and Cued Selective Reminding Test. Attention‐domain composite scores were calculated using the Digit Span of WAIS‐III. Executive function/processing speed domain composite scores were calculated using the Trail‐Making Test, the Digit Symbol Substitution Test of the WAIS‐III, and the Letter Word Fluency Test. WAIS‐III, Wechsler Adult Intelligence Scale.
TABLE 2.
Primary and secondary cognitive endpoints from baseline to 18 months, modified intention‐to‐treat population.
| Intervention | Control | Difference between intervention and control groups | |||
|---|---|---|---|---|---|
| Cognitive composite score | Mean change (SE) | Mean change (SE) | Estimate (95% CI) | p value | Cohen's d |
| Primary | |||||
| Global composite score | 0.55 (0.04) | 0.39 (0.04) | 0.16 (0.04 to 0.27) | 0.009 | 0.38 |
| Post hoc analysis | |||||
| Attention | 0.32 (0.09) | 0.20 (0.09) | 0.12 (−0.10 to 0.35) | 0.28 | 0.16 |
| Executive functioning Processing speed | 0.32 (0.06) | 0.16 (0.06) | 0.16 (0.02 to 0.30) | 0.03 | 0.37 |
| Memory | 1.01 (0.07) | 0.83 (0.07) | 0.19 (0.01 to 0.37) | 0.04 | 0.29 |
Notes: The mixed model for repeated measures analyses assessed between‐group differences (group × time interaction) in the modeled changes from baseline to 18 months, based on data from all participants with at least one post‐baseline measurement. Scores on the global composite score, attention, executive functioning/processing speed, and memory‐domain scores were estimated as mean values (standard errors) of z‐scores of the cognitive tests included in each cognitive outcome, with higher scores indicating better performance. A positive value of the estimate of between‐group differences indicates that the effect favors the intervention group.
Abbreviations: CI, confidence interval; SE, standard error.
Among the secondary endpoints, the frailty states at 18 months were as follows: physical frailty (intervention vs control: 1.1% vs 1.2%, p > 0.99), social frailty (27.6% vs 41.2%, p = 0.07), and oral frailty (34.5% vs 32.9%, p = 0.10) (included in Table S4). In addition, for the other secondary outcome, the digital cognitive tests (Cogstate Brief Battery; Cogstate, Melbourne, Australia), the composite score consisting of the reaction speed and attention scores (101.17 [SE 0.82] vs 97.84 [0.84]; p < 0.01) showed a significant difference between the groups (Table 3). , , ,
TABLE 3.
Change from baseline to 18 months in Cogstate Brief Battery.
| Score of the Cogstate Brief Battery | Intervention | Control | |||
|---|---|---|---|---|---|
| Cognitive endpoint | Time | Mean (SE) | 95% CI | Mean (SE) | 95% CI |
| Composite Score A | Baseline | 98.51 (0.75) | 97.04 to 99.98 | 98.51 (0.74) | 97.05 to 99.97 |
| 18 months | 101.17 (0.82) | 99.55 to 102.79 | 97.84 (0.84) | 96.18 to 99.49 | |
| Composite Score B | Baseline | 95.29 (1.45) | 93.03 to 97.55 | 93.85 (1.14) | 91.60 to 96.10 |
| 18 months | 99.06 (1.21) | 96.67 to 101.45 | 96.95 (1.24) | 94.51 to 99.39 | |
Abbreviations: CI, confidence interval; Composite Score A, composite scores of reaction speed and attention; Composite Score B, composite scores of visual learning and memory; SE, standard error.
The changes from baseline to 18 months in the post hoc analysis showed significant differences in the FTSST (p = 0.002) and PEmax (p = 0.04) (included in Table S5). There were no apparent differences in blood test values or ADL scores between groups (Tables S6 and S7).
Adherence to the intervention components (with or without participation) was high except for cognitive training using a tablet (nutritional counseling, n = 91 [97.8%]; physical exercise, n = 91 [97.8%]; cognitive training, n = 16 [17.2%]; recording and group work to monitor vascular risk factors, n = 91 [97.8%]). The average participation rate for all 78 group exercises was 87.8% (SD 3.4%, Table 4).
TABLE 4.
Data on recorded adherence in intervention components.
| Number of sessions | Participants (%) |
|---|---|
| Nutritional intervention, individual interview: > two times | |
| Yes | 97.8 |
| No | 2.2 |
| Nutritional intervention, telephone interview: > seven times | |
| Yes | 98.9 |
| No | 1.1 |
| Exercise training, group exercise: > 75% participation rate | |
| Yes | 97.8 |
| No | 2.2 |
| Average participation rate in all sessions | 87.8 |
| Cognitive training, use of applications: > 1 h/week | |
| Yes | 17.2 |
| No | 82.8 |
| Monitoring of vascular/metabolic factors: > 75% participation rate | |
| Yes | 97.8 |
| No | 2.2 |
Note: Data on dropouts during the process are excluded.
Few adverse events that could have resulted from study participation were reported, with the most common being falls (two [2.0%] participants in the intervention group). No serious adverse events related to the intervention were reported, and no hospitalization was required (Table 5).
TABLE 5.
Self‐reported adverse events during study.
| Events |
Total (n = 203) |
Intervention (n = 101) |
Control (n = 102) |
|---|---|---|---|
| All adverse events | 90 (44.3, 37.4 to 51.5) | 46 (45.5, 35.6 to 55.8) | 44 (43.1, 33.4 to 53.3) |
| Regarding causal link with study | |||
| Clearly relevant | 2 (1.0, 0.1 to 3.5) | 2 (2.0, 0.2 to 7.0) | 0 (0, 0 to 3.6) |
| Possibly relevant | 2 (1.0, 0.1 to 3.5) | 2 (2.0, 0.2 to 7.0) | 0 (0, 0 to 3.6) |
| Not related | 86 (42.4, 35.5 to 49.5) | 42 (41.6, 31.9 to 51.8) | 44 (43.1, 33.3 to 53.3) |
| List of adverse events | |||
| Fall | 24 (11.8, 7.7 to 17.1) | 17 (16.8, 10.1 to 25.6) | 7 (6.9, 2.8 to 13.6) |
| Musculoskeletal pain | 18 (8.9, 5.3 to 13.7) | 6 (5.9, 2.2 to 12.5) | 12 (11.8, 6.2 to 19.6) |
| Fracture | 7 (3.4, 1.4 to 7.0) | 7 (6.9, 2.8 to 13.8) | 0 (0, 0 to 3.6) |
| Stroke | 3 (1.5, 0.03 to 2.7) | 2 (2.0, 0.2 to 7.0) | 1 (1.0, 0.02 to 5.3) |
| Subarachnoid hemorrhage | 1 (0.5, 0.01 to 2.7) | 0 (0, 0 to 3.6) | 1 (1.0, 0.02 to 5.3) |
| Parkinson's disease | 1 (0.5, 0.01 to 2.7) | 0 (0, 0 to 3.6) | 1 (1.0, 0.02 to 5.3) |
| Cancer | 8 (3.9, 1.7 to 7.6) | 4 (4.0, 1.1 to 9.8) | 4 (3.9, 1.1 to 9.7) |
| COVID‐19 | 1 (0.5, 0.01 to 2.7) | 0 (0, 0 to 3.6) | 1 (1.0, 0.02 to 5.3) |
| Medical disorder | 11 (5.4, 2.7 to 9.5) | 4 (4.0, 1.1 to 9.8) | 7 (6.9, 2.8 to 13.6) |
| Other | 18 (8.9, 5.3 to 13.7) | 7 (6.9, 2.8 to 13.8) | 11 (10.8, 5.5 to 18.5) |
| Death during study | 4 (2.0, 0.05 to 5.0) | 2 (2.0, 0.2 to 7.0) | 2 (2.0, 0.2 to 6.9) |
Note: Diagnoses were made between baseline visit and June 9, 2022. Data are presented as n (%; 95% confidence interval).
4. DISCUSSION
This study showed that an 18‐month multifactorial intervention for preventing dementia improved the cognitive function composite score in the intervention group compared to the control group. Serious adverse events from the intervention were rare, and participant dropout rates were low, confirming the safety of the study and that it was conducted according to the study objectives. The results support the findings of three previous large‐scale, high‐quality studies conducted in recent years, the FINGER study, 6 the Taiwan Health Promotion Intervention Study for Elders (THISCE), 8 and the Taiwan Integrated Geriatric Care (TIGER) study. 9 Although it is widely known that lifestyle‐related diseases such as hypertension and diabetes, physical inactivity, and poor nutritional status are risk factors for dementia, 5 our study shows that these factors can be overcome by multifactorial interventions probably due to increasing cognitive reserve, 30 which may prevent or delay the onset of dementia. The study was also successful in showing that multifactorial interventions are effective in maintaining and improving cognitive function across cultures and practices. While some previous reports, including the present study, have been able to prove the effects of the interventions described above, others have failed to do so. 31 Although certain conclusions have not yet been reached, we considered that it would be difficult to prove efficacy unless the right combination of the right subject, the right intervention and the right evaluation were achieved, and in view of the FINGER 6 and MAPT 32 studies, this study may have been particularly successful in targeting elderly people with vascular risk factors.
The post hoc analyses showed that the intervention group had improved executive function/processing speed and memory function among the domain‐specific cognitive function composite scores compared with the control group. The improvement in the executive function/processing speed domain was consistent with the result of the main secondary analysis of the FINGER study. In addition, to the best of our knowledge, no previous studies reported the effect of a multifactorial intervention for dementia prevention on the improvement of composite scores in memory domains. We believe that the main reason for improving memory function is that our intervention included “Cognicise,” a dual‐task training program developed in Japan that combines physical and cognitive training. A previous study reported that MMSE and Logical Memory II scores improved in participants who performed Cognicise for 40 weeks. 33 In the present study, participation in the weekly 90‐min intervention, including 20‐min Cognicise, remained high, which may have improved cognitive function in many domains. The physical exercise program that occupied most of the intervention time in this study included stretching, strength training, and aerobic exercise, 34 , 35 but also the Cognicise. 33 It has been reported that combining cognitive and physical training can improve cognitive performance more than either alone. 36 , 37 , 38 , 39 , 40 Implementing cognitive training alone may burden subjects psychologically. 41 , 42 , 43 The tablet‐based home cognitive training implementation rate gradually decreased during the entire study period. In contrast, adherence to physical exercise, including the dual‐task exercise, was high, suggesting that these exercises are practical.
Despite the relatively short intervention period of 18 months, the mean (95% CI) difference in global cognitive composite scores between the groups in this study was 0.16 (0.04 to 0.27), which is greater than the 0.03 (0.01 to 0.06) obtained in a recent meta‐analysis 44 of three studies. We believe that our interventions including Cognicise and their high adherence have had a positive impact.
In this study, the Cogstate Brief Battery, a tablet‐based cognitive evaluation, showed a significant difference in the composite score of the reaction speed and attention scores in the intervention group. The results were similar to the primary outcomes, suggesting that the tablet‐based Cogstate Brief Battery can demonstrate intervention effects while reducing the human burden and that the Cogstate Brief Battery may be a useful evaluation tool for the social implementation of dementia prevention in the future.
An analysis of physical functions revealed that there were significant differences in the intervention group in the FTSST and PEmax from baseline to 18 months. The FTSST is commonly used to assess lower extremity functional strength, transitional movements, balance, and fall risk in older people. 45 The positive results on lower extremity muscle strength and respiratory muscle strength suggested that our program, including aerobic and resistance exercise, was enough for older people in the intervention group to improve their physical functions. 46 The mechanism and extent to which the enhancement of respiratory muscle strength contributes to the improvement of cognitive function are still unclear, and further research explicitly focusing on the relationship between aerobic exercise, respiratory muscle strength, and cognitive function in older people may provide additional insight.
Although Japan was in the middle of a COVID‐19 pandemic situation during this study, the number of COVID‐19 cases in Tamba was low because of its rural location, and we continued our face‐to‐face group‐based intervention throughout this study, which was very fortunate and advantageous. COVID‐19 restrictions have been reported to cause adverse lifestyle changes, including feelings of loneliness, sleep disturbance, and reduced physical activity. 47 , 48 In exercise interventions for older people, the effects may be enhanced by mutual influence among participants. 49 A cluster randomized trial showed that group‐based resistance and balance training could improve older people's balance confidence and ability more than home‐based or walking programs. 50 Unsurprisingly, participation in group‐based interventions can also ameliorate social isolation, 51 , 52 , 53 , 54 which is a risk factor for dementia. We not only included time for group discussions during our weekly interventions but also allowed participants to interact with each other at any time via the social media we had set up. We believed that we could promote positive interactions among participants, which resulted in their enjoying the intervention, achieving high participation rates (average participation rate in all sessions: 87.8%), and achieving high efficacy.
Although there were significant group differences in the change in the global cognitive composite score, the score improved not only in the intervention but also in the control group. Similar trends were observed in the FINGER study and other studies. In this study, the control group received only health‐related pamphlets, and no other information was provided. The increase in the global cognitive composite score in the control group may be due to a specific learning effect on each neuropsychological test and/or to behavioral changes that contribute to maintaining or improving cognitive function caused by just participating in this study. Ultimately, the intervention group showed better results than the control group, and there is no doubt that the intervention effect outweighs the improvement in the control group.
The strengths of this study are that it demonstrated significant cognitive improvement effects of the intervention based on a well‐designed protocol, a supervised exercise program, high adherence rates, and the use of validated outcome measures. However, this study has several limitations. First of all, this was a single‐center study conducted in a rural area, and we have yet to validate the effectiveness of our methodology in urban populations of Japan. We hope to clarify this through a meta‐analysis of the results of the J‐MINT study, which conducted a similar intervention in urban areas. Second, although our study protocol was based on the FINGER methodology, the outcome measures of the neuropsychological tests used in both studies were not completely consistent. In the secondary analysis, the FINGER study analyzed the three domains of executive functioning, processing speed, and memory, whereas we analyzed attention, executive function/processing speed, and memory. The assignment of each neuropsychological test in the areas of cognitive function that best reflect it should be thoroughly discussed in the future. Third, although most of our interventions were successful, adherence to cognitive training using tablets at home was low. The percentage of participants who used the device at a frequency of 1 to 60 min/week was 55.9% through regular monitoring of use, advice on improving their efforts, and encouragement to users during the weekly face‐to‐face intervention. However, only 17.2% of the participants used the device for 61 min or more per week, which was our target, suggesting that the intervention needed to be improved. In the next opportunity, we may need to reexamine our interventions to consider differences in participants’ skills and knowledge of electronic devices. Last, head magnetic resonance imaging and biomarker blood sampling were not sufficiently performed on the subjects in this study, and it is currently unclear what brain pathology was actually present. We hope that this can be clarified in future follow‐up studies.
In conclusion, community‐dwelling older adults at high risk of dementia had improved cognitive composite scores with an 18‐month multifactorial non‐pharmacological intervention consisting of lifestyle‐related disease management, physical exercise, nutritional counseling, and cognitive training. Serious adverse events were rare, and dropout rates were low, demonstrating the safety and effectiveness of the intervention in this study for older people at future risk of dementia.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. Author disclosures are available in the Supporting Information.
CONSENT STATEMENT
The J‐MINT PRIME Tamba research plan was reviewed and approved by the Kobe University Ethics Committee for Health Sciences (Approval No.: 922). All participants were fully informed about the study, understood the benefits and potential risks of participation, and provided written informed consent.
Supporting information
Supporting Information
Supporting Information
Supporting Information
ACKNOWLEDGMENTS
We are grateful to the participants and staff of the National Center for Geriatrics and Gerontology, the Tamba City Health Section, the Tamba City Medical Association, the Hyogo Prefectural Tamba Medical Centre, SOMPO Care Inc., and J‐MINT PRIME Tamba team. This research was supported by AMED (Grant No. JP20de0107002) and JSPS KAKENHI (Grant No. JP20K19241).
Oki Y, Osaki T, Kumagai R, et al. An 18‐month multimodal intervention trial for preventing dementia: J‐MINT PRIME Tamba. Alzheimer's Dement. 2024;20:6972–6983. 10.1002/alz.14170
REFERENCES
- 1. WHO . Global Status Report on the Public Health Response to Dementia. WHO Press; 2021. [Google Scholar]
- 2. Cabinet Office of Japan . Annual Report on the Aging Society; 2017. https://www8.cao.go.jp/kourei/english/annualreport/2017/pdf/cover.pdf [Google Scholar]
- 3. Cummings J, Zhou Y, Lee G, Zhong K, Fonseca J, Cheng F. Alzheimer's disease drug development pipeline: 2023. Alzheimers Dement. 2023;9(2):e12385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in early Alzheimer's disease. N Engl J Med. 2023;388:9‐21. [DOI] [PubMed] [Google Scholar]
- 5. Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396:413‐446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ngandu T, Lehtisalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at‐risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385:2255‐2263. [DOI] [PubMed] [Google Scholar]
- 7. Kivipelto M, Mangialasche F, Snyder HM, et al. World‐Wide FINGERS Network: a global approach to risk reduction and prevention of dementia. Alzheimers Dement. 2020;16:1078‐1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen L‐K, Hwang A‐C, Lee W‐J, et al. Efficacy of multidomain interventions to improve physical frailty, depression and cognition: data from cluster‐randomized controlled trials. JCSM. 2020;11:650‐662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee W‐J, Peng L‐N, Lin C‐H, et al. Effects of incorporating multidomain interventions into integrated primary care on quality of life: a randomised controlled trial. Lancet Healthy Longev. 2021;2:e712‐e723. [DOI] [PubMed] [Google Scholar]
- 10. Sugimoto T, Sakurai T, Akatsu H, et al. The Japan‐multimodal intervention trial for prevention of dementia (J‐MINT): the study protocol for an 18‐month, multicenter, randomized, controlled trial. J Prev Alzheimers Dis. 2021;8:465‐476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kumagai R, Osaki T, Oki Y, et al. The Japan‐multimodal intervention trial for prevention of dementia PRIME Tamba (J‐MINT PRIME Tamba): study protocol of a randomised controlled multi‐domain intervention trial. Arch Gerontol Geriatr. 2023;104:104803. [DOI] [PubMed] [Google Scholar]
- 12. Awata S, Sugiyama M, Ito K, et al. Development of the dementia assessment sheet for community‐based integrated care system. Geriatr Gerontol Int. 2016;16:123‐131. [DOI] [PubMed] [Google Scholar]
- 13. Folstein MF, Folstein SE, McHugh PR. “Mini‐mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189‐198. [DOI] [PubMed] [Google Scholar]
- 14. Committee report: glycemic targets for elderly patients with diabetes: Japan diabetes society (JDS)/Japan geriatrics society (JGS) joint committee on improving care for elderly patients with diabetes. J Diabetes Investig. 2017;8(1):126‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Umemura S, Arima H, Arima S, et al. The Japanese Society of Hypertension guidelines for the management of hypertension (JSH 2019). Hypertens Res. 2019;42(9):1235‐1481. [DOI] [PubMed] [Google Scholar]
- 16. Kinoshita M, Yokote K, Arai H, et al. Japan Atherosclerosis Society (JAS) guidelines for prevention of atherosclerotic cardiovascular diseases 2017. J Atheroscler Thromb. 2018;25(9):846‐984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Reynolds CR, Powel J. Wechsler memory scale—revised: David A. Wechsler. New York: the Psychological Corporation. Harcourt Brace Jovanovich, Inc, 1987:150 pp. Arch Clin Neuropsychol. 1988;3:397‐403. [Google Scholar]
- 18. Grober E, Buschke H. Genuine memory deficits in dementia. Dev Neuropsychol. 1987;3:13‐36. [Google Scholar]
- 19. Wechsler D. Manual for the Wechsler Adult Intelligence Scale. 4th ed. Psychological Corp; 1955. [Google Scholar]
- 20. Lezak MD, Howieson DB, Loring DW, et al. Neuropsychological Assessment. 4th ed. Oxford University Press; 2004. [Google Scholar]
- 21. Mahoney FI, Barthel DW. Functional evaluation: the Barthel index: a simple index of independence useful in scoring improvement in the rehabilitation of the chronically ill. Md State Med J. 1965;14:61‐65. [PubMed] [Google Scholar]
- 22. Lawton MP, Brody EM. Assessment of older people: self‐maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179‐186. [PubMed] [Google Scholar]
- 23. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146‐M156. [DOI] [PubMed] [Google Scholar]
- 24. Makizako H, Shimada H, Tsutsumimoto K, et al. Social frailty in community‐dwelling older adults as a risk factor for disability. J Am Med Dir Assoc. 2015;16(11):1003. e7‐e11. [DOI] [PubMed] [Google Scholar]
- 25. Tanaka T, Hirano H, Ohara Y, Nishimoto M, Iijima K. Oral Frailty Index‐8 in the risk assessment of new‐onset oral frailty and functional disability among community‐dwelling older adults. Arch Gerontol Geriatr. 2021;94:104340. [DOI] [PubMed] [Google Scholar]
- 26. Fredrickson J, Maruff P, Woodward M, et al. Evaluation of the usability of a brief computerized cognitive screening test in older people for epidemiological studies. Neuroepidemiology. 2010;34:65‐75. [DOI] [PubMed] [Google Scholar]
- 27. Chiu H‐L, Chu H, Tsai J‐C, et al. The effect of cognitive‐based training for the healthy older people: a meta‐analysis of randomized controlled trials. PLoS One. 2017;12:e0176742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kenward MG, Roger JH. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics. 1997;53(3):983‐997. [PubMed] [Google Scholar]
- 29. Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26:404‐413. [Google Scholar]
- 30. Stern Y. Cognitive reserve in ageing and Alzheimer's disease. Lancet Neurol. 2012;11:1006‐1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Solomon A, Stephen R, Altomare D, et al. Multidomain interventions: state‐of‐the‐art and future directions for protocols to implement precision dementia risk reduction. A user manual for Brain Health Services‐part 4 of 6. Alzheimers Res Ther. 2021;13(1):171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vellas B, Carrie I, Gillette‐Guyonnet S, et al. MAPT study: a multidomain approach for preventing Alzheimer's disease: design and baseline data. J Prev Alzheimers Dis. 2014;1(1):13‐22. [PMC free article] [PubMed] [Google Scholar]
- 33. Shimada H, Makizako H, Doi T, et al. Effects of combined physical and cognitive exercises on cognition and mobility in patients with mild cognitive impairment: a randomized clinical trial. J Am Med Dir Assoc. 2018;19:584‐591. [DOI] [PubMed] [Google Scholar]
- 34. Bisbe M, Fuente‐Vidal A, López E, et al. Comparative cognitive effects of choreographed exercise and multimodal physical therapy in older adults with amnestic mild cognitive impairment: randomized clinical trial. J Alzheimers Dis. 2020;73(2):769‐783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Smith PJ, Blumenthal JA, Hoffman BM, et al. Aerobic exercise and neurocognitive performance: a meta‐analytic review of randomized controlled trials. Psychosom Med. 2010;72(3):239‐252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shatil E. Does combined cognitive training and physical activity training enhance cognitive abilities more than either alone? A four‐condition randomized controlled trial among healthy older adults. Front Aging Neurosci. 2013;5:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mancioppi G, Fiorini L, Rovini E, Cavallo F. The use of motor and cognitive dual‐task quantitative assessment on subjects with mild cognitive impairment: a systematic review. Mech Ageing Dev. 2021;193:111393. [DOI] [PubMed] [Google Scholar]
- 38. Gallou‐Guyot M, Mandigout S, Bherer L, Perrochon A. Effects of exergames and cognitive‐motor dual‐task training on cognitive, physical and dual‐task functions in cognitively healthy older adults: an overview. Ageing Res Rev. 2020;63:101135. [DOI] [PubMed] [Google Scholar]
- 39. Soares VN, Yoshida HM, Magna TS, Sampaio RAC, Fernandes PT. Comparison of exergames versus conventional exercises on the cognitive skills of older adults: a systematic review with meta‐analysis. Arch Gerontol Geriatr. 2021;97:104485. [DOI] [PubMed] [Google Scholar]
- 40. Lee S, Harada K, Bae S, et al. A non‐pharmacological multidomain intervention of dual‐task exercise and social activity affects the cognitive function in community‐dwelling older adults with mild to moderate cognitive decline: a randomized controlled trial. Front Aging Neurosci. 2023;15:1005410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Colquitt JA, LePine JA, Noe RA. Toward an integrative theory of training motivation: a meta‐analytic path analysis of 20 years of research. J Appl Psychol. 2000;85(5):678‐707. [DOI] [PubMed] [Google Scholar]
- 42. Wouters P, Van Nimwegen C, Van Oostendorp H, Van Der Spek ED. A meta‐analysis of the cognitive and motivational effects of serious games. J Educ Psychol. 2013;105(2):249‐265. [Google Scholar]
- 43. Lampit A, Hallock H, Valenzuela M. Computerized cognitive training in cognitively healthy older adults: a systematic review and meta‐analysis of effect modifiers. PLOS Med. 2014;11(11):e1001756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hafdi M, Hoevenaar‐Blom MP, Richard E. Multi‐domain interventions for the prevention of dementia and cognitive decline. Cochrane Database Syst Rev. 2021;11:CD013572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Annweiler C, Schott AM, Abellan Van Kan G, Rolland Y, Blain H, Fantino B. The Five‐Times‐Sit‐to‐stand test, a marker of global cognitive functioning among community‐dwelling older women. J Nutr Health Aging. 2011;15:271‐276. [DOI] [PubMed] [Google Scholar]
- 46. Gea J, Ausín P, Martínez‐Llorens JM, Barreiro E. Respiratory muscle senescence in ageing and chronic lung diseases. Eur Respir Rev. 2020;29:200087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Waterink L, Bakker ED, Visser LNC, et al. Changes in brain‐health related modifiable risk factors in older adults after one year of COVID‐19‐restrictions. Front Psychiatry. 2022;13:877460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Siette J, Dodds L, Brooks C, Deckers K. Older adults’ perspectives towards optimizing lifestyle behaviors and strategies to support healthy brain ageing during COVID‐19 restrictions. Front Public Health. 2023;11:1205001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. James BD, Wilson RS, Barnes LL, Bennett DA. Late‐life social activity and cognitive decline in old age. J Int Neuropsychol Soc. 2011;17:998‐1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cyarto EV, Brown WJ, Marshall AL, Trost SG. Comparative effects of home‐ and group‐based exercise on balance confidence and balance ability in older adults: cluster randomized trial. Gerontology. 2008;54:272‐280. [DOI] [PubMed] [Google Scholar]
- 51. Donovan NJ, Blazer D. Social isolation and loneliness in older adults: review and commentary of a National Academies report. Am J Geriatr Psychiatry. 2020;28:1233‐1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Das Gupta D, Kelekar U, Rice D. Associations between living alone, depression, and falls among community‐dwelling older adults in the US. Prev Med Rep. 2020;20:101273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Huang AR, Roth DL, Cidav T, et al. Social isolation and 9‐year dementia risk in community‐dwelling Medicare beneficiaries in the United States. J Am Geriatr Soc. 2023;71(3):765‐773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Shen C, Rolls ET, Cheng W, et al. Associations of social isolation and loneliness with later dementia. Neurology. 2022;99(2):e164‐e175. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information
Supporting Information


