Abstract
To examine the early events of the life cycle of human immunodeficiency virus type 1 (HIV-1), we analyzed the intracellular complexes mediating reverse transcription isolated from acutely infected cells. Partial purification of the reverse transcription complexes (RTCs) by equilibrium density fractionation and velocity sedimentation indicated that two species of RTCs are formed but only one species is able to synthesize DNA. Most of the capsid, matrix, and reverse transcriptase (RT) proteins dissociate from the complex soon after cell infection, but Vpr remains associated with the RTC. The RTCs isolated 1, 4, and 7 h after infection are competent for reverse transcription in vitro, indicating that a small proportion of RT remains associated with them. HIV RTCs isolated early after infection have a sedimentation velocity of approximately 560S. Later, different species with a sedimentation velocity ranging from 350S to 100S appear. Nuclear-associated RTCs have a sedimentation velocity of 80S. Shortly after initiation of reverse transcription, the viral strong-stop DNA within the RTC is sensitive to nuclease digestion and becomes protected when reverse transcription is almost completed.
All retroviruses copy the RNA genome to form a double-stranded DNA molecule that is subsequently integrated into the host chromosomal DNA. The process is catalyzed by reverse transcriptase (RT) protein and is mainly carried out in the cytoplasm soon after viral penetration into the cell (30). Reverse transcription of the viral RNA genome into DNA is generally completed in 8 to 12 h. We have recently characterized the Moloney murine leukemia virus (MoMLV) intracellular viral complex mediating reverse transcription and found that at least three distinct species of reverse transcription complexes (RTCs) are formed shortly after acute infection (7). Only one of these species is able to start and complete reverse transcription. This RTC contains at least the viral genome, p30 capsid (CA), integrase (IN), and RT proteins, and its sedimentation velocity decreases with time, suggesting that a stepwise uncoating process may occur during reverse transcription.
Little is known about the structure and composition of the human immunodeficiency virus type 1 (HIV-1) RTC, particularly during the early steps after virus internalization. Electron microscopic studies showed that HIV cores are disrupted shortly after virus-cell fusion, in contrast to MLV cores, which persist longer (15, 27). The viral RNA and associated proteins are then released into the cytoplasm and are likely to interact with the cytoskeleton (1). At later stages of the viral life cycle, the fully reverse transcribed HIV-1 DNA appears to be associated with RT, IN, and a subset of phosphorylated matrix (MA) but only very weakly with p24 CA proteins (4, 6, 18, 23). Specific cellular proteins have also been found to associate with the viral DNA, including those increasing the efficiency of integration of the viral genome in vitro (5) and others preventing self-integration of the viral DNA which would result in an nonproductive infection (19).
HIV, unlike MLVs, can infect nondividing cells (20, 28). This ability depends on the active nuclear transport of its genome into the nucleus of infected cells (2). Although a variety of models have been proposed (3, 12, 13, 17, 32, 35), there is no consensus on the precise mechanisms regulating nuclear import of HIV (9, 11). Interactions between the intracellular viral complexes mediating reverse transcription and karyopherins or the nuclear pore complex may play a role (10, 13, 17, 26, 32). A better characterization of the organization of HIV RTC may advance understanding of the early interactions between virus and infected cells as well as the mechanisms regulating HIV-1 infection of nondividing cells. To examine the dynamics of the early events in the HIV-1 life cycle, we have analyzed detergent-free cytoplasmic and nuclear extracts at various time points after acute infection and characterized the RTCs by equilibrium density fractionation and velocity sedimentation.
MATERIALS AND METHODS
Cells and viruses.
293T and HeLa cells were grown in Dulbecco's modified Eagle's medium supplemented with 2 mM glutamine and 10% fetal calf serum at 37°C in an atmosphere containing 5% CO2. To make replication-incompetent HIV-1 vectors, 5 × 106 293T cells were seeded in 100-mm-diameter dishes and transfected the following day by calcium phosphate with 20 μg of plasmid pHR' (containing the green fluorescent protein cDNA), 15 μg of plasmid pCMVΔR9, and 5 μg of plasmid pMD.G (25) per dish. Culture medium was replaced after 24 h, the virus-containing supernatant was collected 48 h after transfection and filtered through a 0.45-μm-pore-size filter. The filtered supernatant was incubated at 37°C for 1 h in the presence of 70 U of DNase I (Boehringer) per ml and 10 mM MgCl2. Virus was purified by centrifugation through a 25 to 45% sucrose cushion at 23,000 rpm in a Beckman SW28 rotor for 2 h at 4°C. The sucrose interphase containing purified virions was diluted fivefold in Dulbecco's modified Eagle medium containing 20 mM HEPES (pH 7.4) and frozen at −80°C. Viral titers were determined by infecting HeLa cells with serial dilution of virus containing supernatant in the presence of Polybrene (8 μg/ml). Approximately 36 h after infection, cells were harvested in phosphate-buffered saline (PBS) containing 10 mM EDTA and analysed by fluorescence-activated cell sorting to detect green fluorescent protein expression.
Cell extracts.
Approximately 107 HeLa cells were infected in 175-cm2 flasks with 30 ml of 1:1-diluted virus stock (3 × 106 CFU/ml) in the presence of Polybrene (8 μg/ml). Infected cells were rapidly cooled to 4°C and incubated for 2 h to allow viral adhesion to the cell receptor but not viral internalization. Cells were then incubated at 37°C for 1, 4, 7, and 16 h, washed once in PBS–0.5 mM EDTA, trypsinized, and washed again with PBS. All subsequent manipulations were carried out at 4°C. The pellet containing the infected cells was resuspended in 5 volumes of hypotonic buffer (10 mM HEPES [pH 7.9], 1.5 mM MgCl2, 10 mM KCl, 5 mM dithiothreitol [DTT], 20 μg of aprotinin/ml, 2 μg of leupeptin/ml) and centrifuged for 5 min at 5,000 rpm in an Eppendorf centrifuge (model 5415C). The pellet was resupended in 3 volumes of hypotonic buffer and incubated for 10 min. Cells were homogenized with 10 to 15 strokes in a Dounce homogenizer, and nuclei and unbroken cells were pelleted by centrifugation at 3,300 × g for 15 min. The supernatant (called cytoplasmic extract) was clarified by centrifugation at 7,500 × g for 20 min, and the pellet was discarded. The nuclear pellet from the previous centrifugation was resuspended in approximately 600 μl of isotonic buffer (10 mM Tris HCl [pH 7.4], 160 mM KCl, 5 mM MgCl2, 1 mM DTT, 20 μg of aprotinin/ml, 2 μg of leupeptin/ml) and homogenized in a ball-bearing homogenizer. The homogenate was then centrifuged at 7,500 × g for 20 min, and the supernatant (called nuclear extract) was collected. Nuclear and cytoplasmic extracts were adjusted to 8% sucrose, snap-frozen in liquid N2, and stored at −70°C.
Equilibrium density gradients.
Continuous linear sucrose gradients (5 ml) were poured with a two-chamber Hoefer SG gradient maker using 20% sucrose solution in hypotonic buffer and 70% sucrose solution in D2O and kept on ice. The pH of D2O was adjusted to 7.4 by dropwise addition of 10 mM NaOH. Gradients were overlaid with 0.5 ml of cytoplasmic or nuclear extracts and centrifuged at 35,000 rpm at 4°C for 20 h in a Beckman SW55 rotor. Gradients were fractionated by puncturing the bottom of the tube and collecting 12 fractions. The density was calculated by weighing 100 μl of each fraction.
Sedimentation velocity gradients.
Continuous gradients were poured as described above, using 5 and 20% sucrose solutions in 50 mM sodium phosphate buffer (pH 7.4) containing 2 mM DTT, 20 μg of aprotinin/ml, and 2 μg of leupeptin/ml. Approximately 150 μl of the equilibrium density fraction containing the peak of the viral DNA was diluted with 1.2 ml of hypotonic buffer, loaded onto a Centricon 50 concentrator (Amicon), and centrifuged at 4,000 × g for 30 min at 4°C in a Sorvall centrifuge. The concentrate (50 μl) was resuspended in 300 μl of 50 mM sodium phosphate buffer, loaded on a 5 to 20% continuous sucrose gradient, and centrifuged at 23,000 rpm for 1 h at 4°C in a Beckman SW55 rotor. Fractions (0.4 ml each) were collected by puncturing the bottom of the tube, and the density was measured by weighing 100 μl of each fraction. In some instances, viral DNA in fractions was precipitated by adding 1.5 μg of glycogen, sodium acetate to a final concentration of 300 mM, and 2.5 volumes of 100% ethanol. The S value was calculated by the method of McEwen (22). Calibration of the system was performed by independently running 35S-labeled poliovirus, intact MoMLV, and naked viral DNA through identical sucrose gradients.
PCR.
PCR was performed in a final volume of 50 μl containing 1× PCR buffer, 100 μM each deoxynucleoside triphosphate (dNTP), 2.5 mM MgCl2, 5 U of Taq polymerase (Perkin-Elmer), and 30 picomol of each primer. Primer sequences were as follows (nucleotide positions according to Muesing et al. [24] are given in parentheses): strong-stop forward primer, 5′-GGCTAACTAGGGAACCCACTG-3′ (505 to 525); strong-stop reverse complementary primer, 5′-CTGCTAGAGATTTTCCACACTGAC-3′ (630 to 653); Pol forward primer, 5′-TTCTTCAGAGCAGACCAG-3′ (2151 to 2168); Pol reverse complementary primer, 5′-ACTTTTGGGCCATCCATT-3′ (2656 to 2639); positive-strand forward primer, 5′-ACAAGCTAGTACCAGTTGAGCCAGATAAG-3′ (158 to 186); and positive-strand reverse complementary primer, 5′-GCCGTGCGCGCTTCAGCAAGC-3′ (730 to 709). Aliguots of 5 μl of the equilibrium density fractions or 1.5 μl of the sedimentation velocity fractions were used as template for the PCR. Cycle parameters were 94°C for 3 min (first cycle), 94°C for 1 min, 55°C for 30 s, and 68°C for 1 min (35 to 45 cycles), and one final extension cycle at 68°C for 10 min. PCR products were resolved on a 1% agarose–2% NuSieve gel and visualized by ethidium bromide staining. Quantitative PCR was performed in duplicate in the conditions described above, using twofold serial dilutions of the DNA template and HIV plasmid pHR' (from 1 pg to 1.9 fg). After 30 cycles, PCR bands were quantified by the Kodak Digital Science 1D image analysis software, and the linear region of the reaction was then selected to calculate RTC copy number, assuming that 1 fg of a 7-kb plasmid DNA corresponds to 1.3 × 102 molecules.
Antibodies and Western blotting.
Human antiserum against HIV-1 was a gift from Johnson Mak, McFarlane Burnet Centre for Medical Research, Melbourne, Victoria, Australia; a monoclonal antibody against p24 (ABT 14001) was purchased from American Biotechnologies Inc; monoclonal antibody 11G10E6 against HIV RT was a gift from D. Helland, University of Bergen, Bergen, Norway (29); monoclonal antibody 9C5 against HIV-1 matrix was a gift from Bridget Ferns, University College London, London, United Kingdom (8). Antiserum to HIV-1 Vpr (1-46; from Jeffrey Kopp) was obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, National Institutes of Health. Recombinant HIV-1 p17 MA and p24 CA (from S. Adams and G. Reid, respectively) were obtained from the Centralised Facility for AIDS Reagent supported by European Union program EVA (contract QLK2-CT-1 999-00609) and the UK Medical Research Council. For Western blot analyses, 300 μl of each fraction from the density equilibrium gradients was diluted in 1.2 ml of ice-cold 50 mM sodium phosphate buffer (pH 7.4) in the presence of 2 μg of bovine serum albumin (Sigma) per ml and 10% (vol/vol) trichloroacetic acid. Fractions were incubated at −20°C for 16 h and centrifuged for 30 min at 4°C at maximum speed in an Eppendorf Microfuge. Pellets were washed once in a solution of ice-cold 80% acetone in distilled H2O and resuspended in 20 μl of sodium dodecyl sulfate (SDS) loading buffer (0.5 M Tris HCl [pH 6.8], 1% SDS, 10% glycerol, 0.1% bromophenol blue, 1 mM EDTA, 10 mM DTT, 20 μg of aprotinin/ml 2 μg of leupeptin hemisulfate/ml, 10 μg of phenylmethylsulfonyl fluoride). The pH was adjusted to ∼ 7.0 by addition of 1 μl of 1.5 M Tris-HCl (pH 8.8). Samples were boiled for 5 min and loaded on an SDS–12.5% polyacrylamide gel. After polyacrylamide gel electrophoresis (PAGE), the proteins were transferred to a polyvinylidene difluoride (PVDF) membrane (Bio-Rad, Hercules, Calif.) and probed with the primary antibodies for 1 h at room temperature. Horseradish Peroxidase-conjugated secondary antibodies were used diluted 1:10,000 (anti-human; Boehringer) 1:3,000 (anti-mouse; Promega), and 1:10,000 (anti-rabbit; Jackson) in 10% nonfat milk (Nestle, Solon, Ohio). Enhanced chemiluminescence (ECL-Plus; Amersham) was used to develop the blots as described by the manufacturer. Autoradiography films were exposed for different periods of time to ensure linearity of the signal.
Assays for reverse transcription.
RT activity was tested with the oligo(dT)-poly(rA) assay as previously described (14, 31). Briefly, 10 μl of samples was added to 40 μl of RT cocktail {60 mM Tris-HCl (pH 8.0), 180 mM KCl, 6 mM MgCl2, 6 mM DTT, 0.12% Triton X-100, 6 μg of oligo(dT)/ml, 12 μg of poly(rA)/ml, 0.05 mM [α-P32]dTTP (800 Ci/mmol)} for 1 h at 37°C. Samples were spotted onto DE-81 paper and washed three times with 2× SSC (0.3 M NaCl plus 0.03 M sodium citrate). A PhosphorImager (Molecular Dynamics, Sunnyvale, Calif.) was used to quantitate the radioactivity incorporated. Endogenous reverse transcription reactions were carried out in 60 μl of buffer (100 mM Tris-HCl [pH 8.1], 15 mM NaCl, 6 mM MgCl2, 1 mM DTT, 2 mM each dNTP). Fifteen microliters from the density equilibrium fractions was added to the buffer and incubated for 4 to 6 h at 37°C. The products of reverse transcription were detected by PCR using 5 μl of the endogenous reaction as template.
Nuclease treatments
All nuclease treatments were performed in 25 μl isotonic buffer containing 1 mM DTT, 20 μg each of aprotinin and leupeptin hemisulfate per 15 ml, U of S7 micrococcal nuclease (Boehringer), and 2 mM CaCl2. Five microliters from the equilibrium density fractions containing the peak of retroviral DNA was added to the nuclease mixture, and samples were incubated on ice. The reaction was stopped by addition of 4 mM EGTA and kept on ice. Two aliquots of 5 μl each from the same reaction were analyzed by semiquantitative PCR with strong-stop and positive-strand primers. The nuclease sensitivity of the intact RTCs was compared with that of naked viral DNA. Control digests of naked viral DNA were carried out in the presence of 5-μl aliquots from equilibrium density fractions containing cytoplasmic extracts from uninfected HeLa cells and having the same density as the fractions containing the RTCs.
RESULTS
Extraction of the HIV-1 RTC from acutely infected cells.
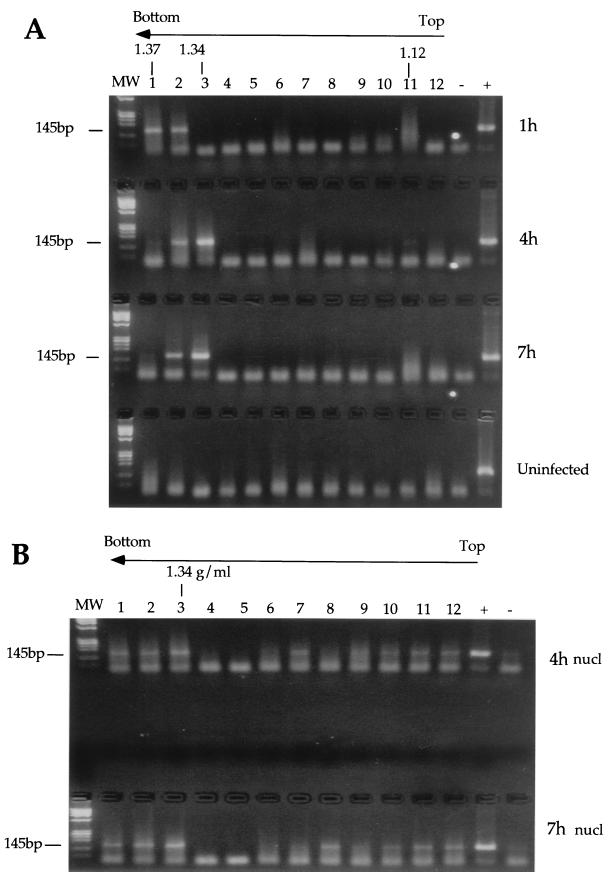
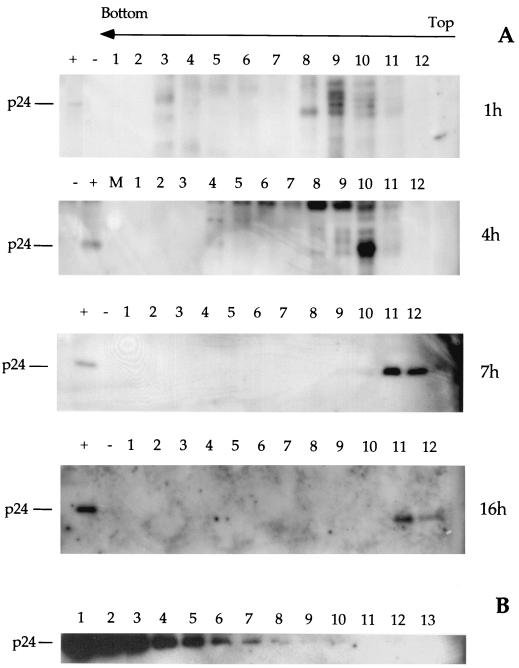
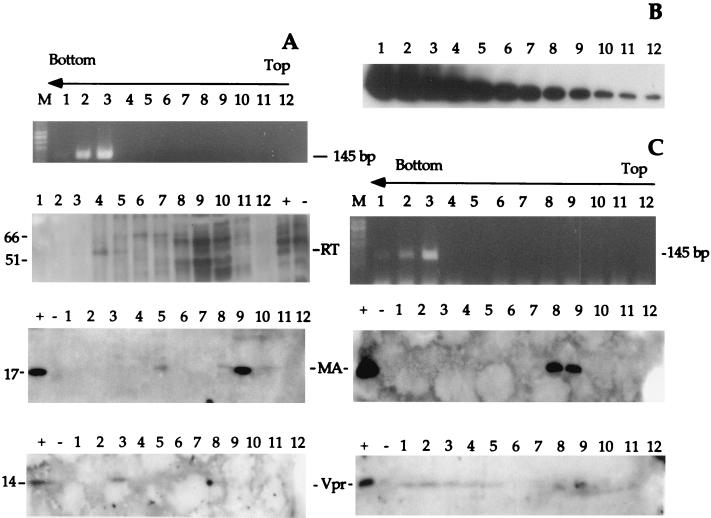
Previous reports suggested that the RTC formed by HIV-1 pseudotyped with vesicular stomatitis virus glycoprotein (VSV-G) envelope can be extracted in mild conditions, in contrast to those formed by wild-type HIV, which can be extracted only in high-ionic-strength buffers (1). To prepare HIV RTCs from acutely infected cells, we used a protocol developed for MoMLV, which allowed the isolation of functionally active RTCs (7). HeLa cells were infected with a recombinant HIV-1 pseudotyped with VSV-G envelope (25) at a multiplicity of infection of 5. Infection was synchronized by preincubating the cells for 2 h at 4°C to allow for virus binding to the receptor. After culturing for various times, cells were trypsinized, washed, and lysed by hypotonic breakage of the cell membrane and Dounce homogenization (see Materials and Methods). The cell extracts were subjected to equilibrium density sedimentation, and fractions were analyzed by PCR and Western blotting. In cytoplasmic extracts, HIV-1 strong-stop DNA could be detected as a discrete peak at a density of 1.34 g/ml, similar to MoMLV strong-stop DNA (7) and consistent with the density of a nucleoprotein complex (Fig. 1). This density did not change significantly with time (Fig. 1 and Table 1). In nuclear extracts, strong-stop DNA was detected also at lower densities though a peak was consistently present at 1.34 g/ml (Fig. 1 and Table 1). This peak was present even after the nuclei were washed in buffer containing 0.5% NP-40 (not shown). The bulk of the p24 protein was detected by both a monoclonal antibody and patient antisera in a discrete peak having a density of 1.14 to 1.12 g/ml and were never found cosedimenting with the viral strong-stop DNA (Fig. 2A). Recombinant HIV p24 was used to test the sensitivity of the Western blotting, and quantitative PCR was used to quantitate the amount of RTC molecules present in the sample. The fraction showing the peak of viral DNA contained normally between 3 × 107 to 5 × 107 RTC molecules/ml (not shown). The limit of detection of p24 by Western blot was approximately 24 pg, corresponding to 6 × 108 molecules (Fig. 2B). Thus, our system was sensitive enough to detect 60 p24 molecules/RTC. This suggested that most CA proteins were lost from the complex after entry into the cell cytoplasm. The quantity of CA in this peak reached a maximum 4 h postinfection. Neither the strong-stop DNA or CA proteins were detected in equilibrium density gradients containing extracts from uninfected cells (not shown). Equilibrium density fractions were analyzed by Western blotting to detect the presence of MA, RT, and Vpr. The peak of MA was found to fractionate at a buoyant density of 1.14 g/ml at 4 and 16 h after acute infection, similar to that for CA (Fig. 3A and C). Small amounts of MA were found at a density of 1.26 g/ml 4 h after infection; however, MA cosedimenting with viral DNA was not detectable (Fig. 3A). Recombinant p17 MA was used to test the sensitivity of the Western blotting. As few as 3.5 pg of p17 could be detected by Western blotting in the same conditions as used to detect p17 in density fractions (Fig. 3B), corresponding to 1.2 × 108 molecules. Thus, the sensitivity of the system allowed visualization of fewer than 15 molecules p17/RTC. RT was also found in the light fractions 4 h after acute infection (Fig. 3A). A discrete peak of Vpr was found cosedimenting with the viral DNA at a density of approximately 1.34 g/ml 4 and 16 h after acute infection, suggesting that substantial levels of Vpr remained associated with the RTC (Fig. 3A and C).
FIG. 1.
PCR analyses of cytoplasmic (A) and nuclear (B) extracts after equilibrium density fractionation using primers specific for the strong-stop DNA (expected band size is 145 bp). HeLa cells were infected with an HIV-1-based vector; cell extracts were prepared 1, 4, and 7 h postinfection, loaded on a 20 to 70% linear sucrose gradient, and centrifuged at 4°C for 20 h at 35,000 rpm in a Beckman SW55 rotor. After centrifugation, gradients were collected in 12 fractions and analyzed. Arrows indicate the direction of the gradient from the lowest (top) to the highest (bottom) density. The density of the fraction containing the peak of the viral DNA is indicated for each time point. Lanes: MW, DNA molecular weight standards; 1 to 12, fractions 1 to 12. The rapidly migrating bands are PCR artifacts. Hirt viral DNA was used as positive control (+); uninfected Hela cells served as a negative control (−). nucl, nuclear.
TABLE 1.
Densities of fractions in which the peak of viral DNA is found
| Time (h) after acute infection | Density (g/ml; mean ± SD) | n |
|---|---|---|
| 1 | 1.35 ± 0.023 | 3 |
| 4 | 1.33 ± 0.021 | 3 |
| 7 | 1.34 | 2 |
| 16 | 1.34 ± 0.05 | 2 |
| 4, nuclear | 1.32 ± 0.025 | 2 |
| 7, nuclear | 1.325 ± 0.025 | 2 |
FIG. 2.
(A) Western blot analyses of equilibrium density fractions containing cytoplasmic extracts collected 1, 4, 7, and 16 h postinfection. Following SDS-PAGE and protein transfer, the PVDF membrane was probed with a monoclonal antibody against HIV p24 capsid protein. Lanes: 1 to 12, fractions 1 to 12 as in Fig. 1; +, purified virus; −, uninfected HeLa cells; M, molecular weight markers. The arrow indicates the direction of the gradient from the lowest (top) to the highest (bottom) density. (B) Testing of sensitivity of Western blotting using recombinant p24. Following SDS-PAGE and protein transfer, the PVDF membrane was probed with the same antibody as used for panel A and for the same exposure time. Lanes 1 to 13 correspond to twofold serial dilutions of recombinant p24 from 3.2 ng to 0.7 pg.
FIG. 3.
(A and C) PCR and Western blot analyses of equilibrium density fractions containing cytoplasmic extracts collected 4 (A) and 16 (C) h after acute infection. Fractions were subjected to PCR with primers specific for the strong-stop DNA (expected size is 145 bp). Lanes: M, DNA molecular weight standards; 1 to 12, fractions 1 to 12. Following SDS-PAGE and protein transfer, the PVDF membrane was probed with a polyclonal antibody against Vpr and a monoclonal antibody against MA and RT. Controls were purified HIV-1 (+) and conditioned medium from untransfected 293T cells (−). Arrows indicate the direction of the gradient, from the lowest density (top) to the highest density (bottom). Positions of molecular weight markers are indicated in kilodaltons on the left. (B) Testing of sensitivity of Western blotting using recombinant p17. Following SDS-PAGE and protein transfer, the PVDF membrane was probed with the same antibody as used for panel A and for the same exposure time. Lanes 1 to 12 correspond to twofold serial dilutions of recombinant p17 from 8 ng to 3.5 pg.
Partially purified RTCs are able to synthesize DNA.
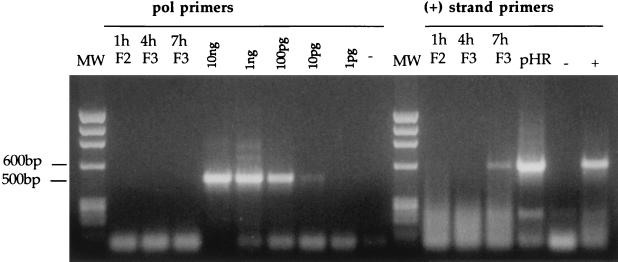
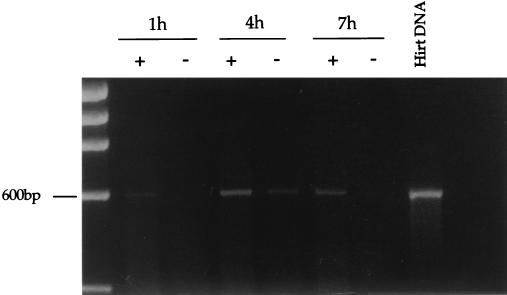
To evaluate whether the fractions containing the strong-stop DNA also contained elongated reverse transcription products, aliquots from the same fractions were subjected to PCR using a forward primer located on the 5′ long terminal repeat U5 region and a reverse primer located in the 5′ untranslated region. These primers are specific for the viral positive-strand DNA, a late reverse transcription product. As shown in Fig. 4, the positive-strand DNA could be detected 7 h after acute infection. Contamination of plasmid DNA carried over from transfection was excluded by PCR amplification of the Pol region in the same fractions. This region is missing in the vector plasmid but is present in the packaging plasmid used to prepare recombinant HIV (25). To test whether the complexes were competent to carry out reverse transcription in vitro, we performed an endogenous reverse transcription assay on 10-fold dilutions of the equilibrium density fractions containing the peak of the strong-stop DNA. Samples were incubated at 37°C in the presence or absence of dNTPs, and aliquots from the reactions were subjected to PCR using primers specific for the positive strand. As shown in Fig. 5, following incubation at 37°C, complexes isolated 1, 4, and 7 h after acute infection were able to synthesize the positive-strand DNA, and addition of exogenous dNTPs increased the efficiency of the reaction. Thus, the complexes sedimenting at 1.34 g/ml are competent for DNA synthesis in vitro. Fractions containing CA and MA proteins but no viral DNA might have contained the viral RNA genome that could not be detected by standard PCR. Therefore an endogenous reverse transcription assay was performed on fractions from the density gradients containing the cytoplasmic extracts collected 1 and 4 h after infection. Only small amounts of strong-stop DNA were detected in some of those fractions containing CA and MA that scored negative in previous assays (not shown). These results suggested that most of the complexes found at lower densities were poorly competent for reverse transcription.
FIG. 4.
PCR analyses of equilibrium density fractions containing cytoplasmic extracts collected 1, 4, and 7 h postinfection. Selected fractions from the equilibrium density gradients shown in Fig. 1 were subjected to PCR using primers specific for the Gag-Pol region (pol primers; expected band size is 500 bp) and for the positive (+)-strand DNA (expected size is 600 bp). Fractions 2 and 3 (F2 and F3) are the same as in Fig. 1. Serial dilutions of pCMVΔR9 plasmid DNA were used as the internal standard for Pol PCR. Hirt DNA from uninfected cells was used as negative control (−). Plasmid pHR' and Hirt DNA from infected cells were used as internal controls for the (+)-strand PCR. MW, DNA molecular weight standards. Low-molecular-weight bands are PCR artifacts.
FIG. 5.
Endogenous reverse transcription assay of equilibrium density fractions containing the peak of the viral DNA from cytoplasmic extracts collected 1, 4, and 7 h postinfection. Samples diluted 1:10 were incubated for 6 h at 37°C in the presence (+) or absence (−) exogenous dNTPs and then subjected to PCR with primers specific for the (+)-strand DNA (expected band size is 600 bp).
Sedimentation velocity of the RTC during reverse transcription.
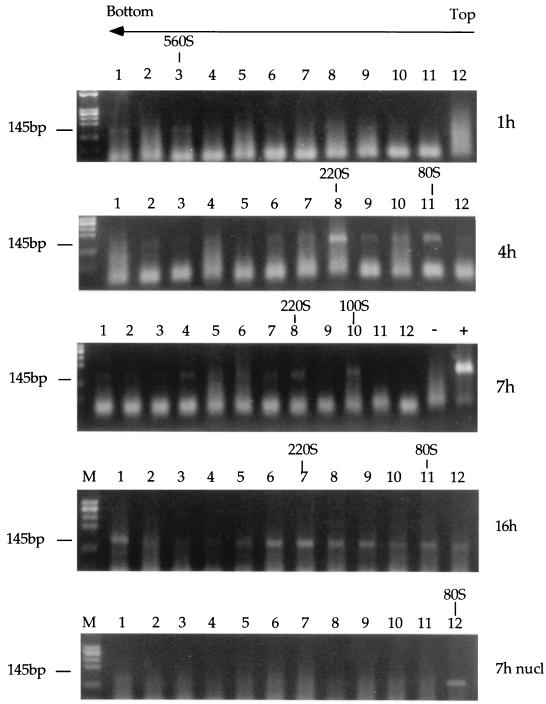
To determine whether there were changes in the size or shape of the RTC with a buoyant density of 1.34 g/ml during reverse transcription, we subjected partially purified RTCs to velocity sedimentation through linear sucrose gradients. The equilibrium density fractions containing the peak of the viral DNA were dialyzed and concentrated using a Centricon filter. The concentrate was then analyzed by sedimentation velocity, and the position of the viral DNA in the gradients was detected by PCR. In the cytoplasmic extracts obtained 1 h after acute infection, a peak of viral DNA with a sedimentation velocity of 560S was found. At later time points, strong-stop DNA was found in many fractions although we consistently detected discrete peaks of viral DNA having sedimentation velocities of 220S and 80S to 100S (Fig. 6 and Table 2); the former species were often more abundant than the latter. In the nuclear extracts, viral DNA was found only in fractions with a sedimentation velocity of approximately 80S (Fig. 6 and Table 2). These results suggest that protein-DNA complexes with a range of sizes and/or shapes are found in the cytoplasm at different times during reverse transcription. Such a range of properties appears much less pronounced in the nuclear-associated complexes.
FIG. 6.
Analysis of RTCs by sedimentation velocity. Cytoplasmic extracts collected 1, 4, 7, and 16 h postinfection and nuclear extracts collected 7 h postinfection were subjected to equilibrium density centrifugation. The fractions containing the peak of the retroviral DNA were further purified, concentrated using a Centricon filter, and centrifuged through a 5 to 20% linear sucrose gradient for 1 h at 23,000 rpm in a Beckman SW55 rotor. Twelve fractions were collected, and the viral DNA in each fraction was detected by PCR using primers specific for the strong-stop DNA (expected band size is 145 bp). The rapidly migrating bands are PCR artifacts. The arrow indicates the direction of the gradient. nucl, nuclear.
TABLE 2.
Sedimentation velocity of HIV-1 RTCsa
| S value(s)b | Time (h) after infection |
|---|---|
| 320 + 240 + 120 | 4 |
| 225 + 100 | 4 |
| 225 + 100 | 4 |
| 225 + 100 | 7 |
| 200 + 100 | 7 |
| 355 + 280 + <80 | 16 |
| 210 + 80 | 16 |
| 80 | 7, nuclear |
| <80 | 7, nuclear |
PCR was performed on fractions collected from velocity sedimentation gradients. Strong-stop DNA was found in different fractions.
Position of each band having the strongest intensity as detected by ethidium bromide staining of the resolving gels.
Sensitivity of the strong-stop DNA to micrococcal nuclease.
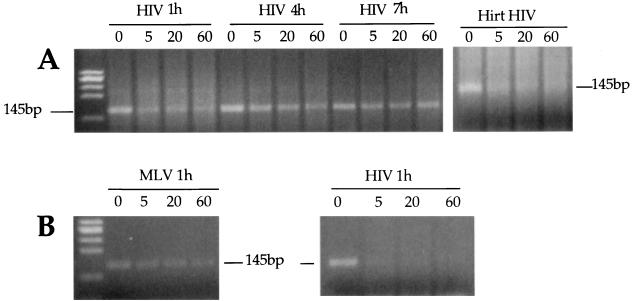
To evaluate the accessibility of the HIV DNA ends within the RTC, we assayed the sensitivity to micrococcal nuclease of the viral strong-stop DNA at various time points after acute infection. The equilibrium density fractions from cytoplasmic extracts containing the peak of the viral DNA were treated with micrococcal nuclease, and the reaction was stopped at the time point indicated in Fig. 7 by addition of EGTA. Nuclease-treated samples were then analyzed by PCR with primers specific for the strong-stop DNA. When naked viral DNA was treated with micrococcal nuclease, the strong-stop DNA appeared to be very sensitive to nuclease digestion. Strong-stop DNA was very sensitive to nuclease digestion in the RTC prepared 1 h after acute infection. To compare HIV and MoMLV, 1-h fractions containing the peak of MoMLV or HIV DNA were mixed 1:1, subjected to nuclease digestion, and analyzed by PCR as described above. While MoMLV strong stop was protected from nuclease digestion, HIV strong stop was not (Fig. 7B). The sensitivity to micrococcal nuclease appeared to decrease in HIV RTCs isolated at later time points (Fig. 7A).
FIG. 7.
Sensitivity of the viral strong-stop DNA within the RTC to micrococcal nuclease digestion. (A) Equilibrium density fractions containing the peak of the viral DNA from the cytoplasmic extracts collected 1, 4, and 7 h postinfection were incubated on ice in the presence of micrococcal nuclease and 2 mM CaCl2. The reactions were stopped by addition of 4 mM EGTA at the indicated time points and analyzed by PCR using primers specific for the strong-stop DNA (expected band size is 145 bp). Naked viral DNA was incubated as above in the presence of micrococcal nuclease and cytoplasmic extracts from uninfected cells (Hirt HIV). (B) Equilibrium density fractions containing the peak of HIV-1 and MoMLV DNAs were mixed 1:1 and subjected to micrococcal nuclease digestion as above. The reaction was analyzed by PCR using primers specific for HIV or MoMLV strong stop. To ensure the linearity of amplification, the samples were subjected to two independent amplification rounds, one of 30 and one of 40 cycles.
DISCUSSION
In this study, we have analyzed the RTC of HIV-1 extracted from cells at times ranging from shortly after viral penetration to completion of reverse transcription. Analyses of the cytoplasmic extracts indicated that the newly synthesized viral DNA sedimented at a density of 1.34 g/ml, similar to that seen for MoMLV (7). In nuclear extracts, viral DNA was found also at lower densities. This is consistent with the possibility that some RTCs remained associated with the nuclear membrane since we did not use detergents to prepare nuclear extracts. In contrast to MoMLV RTC, no p24 CA proteins were found cosedimenting at high density with HIV-1 DNA. CA proteins were instead found in fractions having a buoyant density of approximately 1.14 g/ml. Thus, it is likely that most CA proteins dissociate from HIV RTC very early after infection, although we would have failed to detect any CA if fewer than 60 p24 molecules/RTC were present. Previous electron microscopic studies also indicated that the HIV core is disrupted in the process of virus internalization, while MoMLV cores persist longer inside the infected cells (15, 27). Most RT molecules were also found in the low-density fractions, dissociated from the viral DNA. However, some RT must have been retained within the RTC since complexes isolated 1, 4, and 7 h after acute infection were competent for DNA synthesis in vitro. It would be interesting to understand how small amounts of RT are retained within the RTC. MA was found cosedimenting with CA in the same low-density fractions. Previous studies showed that a subset of phosphorylated MA remains associated with the viral DNA in the preintegration complex (PIC) (2, 3, 4, 12). Our inability to detect MA in the high-density fractions may be due to insufficient sensitivity of the system, although we could detect as few as 15 molecules of p17/RTC (corresponding to about 1% of the MA content of an intact virion), or to the inability of the monoclonal antibody to recognize phosphorylated MA. Vpr was found cosedimenting with the viral DNA, suggesting that it was part of the RTC. This is consistent with reports showing the presence of Vpr in the PIC (12, 16, 26).
RTCs with a buoyant density of 1.34 g/ml were competent for reverse transcription, as indicated by the presence of the (+)-strand DNA in complexes isolated 7 h after acute infection and by their ability to synthesize DNA in vitro. Only small amounts of strong-stop DNA were found in fractions with a density of 1.14 g/ml subjected to an endogenous reverse transcription reaction. Those fractions contained both p24 and MA proteins. The ratio of CA to strong-stop DNA was high in the low-density fractions containing cytoplasmic extracts isolated 1 and 4 h after infection (not shown), indicating that low-density complexes were poorly competent for reverse transcription in vitro. Such complexes did not synthesize strong-stop DNA in vivo 4 h after acute infection and are likely to be dead-end products.
RTC species with a density of 1.34 g/ml appeared to include a wide range of sizes and/or shapes. Complexes isolated 1 h after infection had a sedimentation velocity of 560S, similar to that of early MoMLV RTCs (7). At later stages, complexes with a sedimentation velocity ranging from 350S to between 80S and 100S were observed. Such complexes are slower sedimenting and presumably smaller than MoMLV RTCs isolated at the same time after acute infection, perhaps because no p24 CA remained associated with the HIV RTC. Nuclear-associated RTCs had a sedimentation velocity of approximately 80S, similar to the slow-sedimenting peak observed in cytoplasmic extracts. HIV PICs can be transported actively into the nucleus of nondividing cells, as opposed to MoMLV PICs (2, 21, 28). The smaller size of the cytoplasmic HIV RTC may also play a role in its transport into the nucleus of infected cells.
Another difference between HIV and MoMLV RTCs is the sensitivity of the DNA ends to micrococcal nuclease digestion. While the strong-stop DNA within MoMLV RTC isolated 1, 4, and 7 h after infection is almost completely resistant to nuclease digestion (7), HIV strong-stop DNA is as sensitive as naked DNA in early RTCs and its sensitivity appears to decrease with time. After completion of DNA synthesis, the DNA ends appear to form a large nucleoprotein complex called the intasome (33, 34). Our data suggest that the intasome may take longer to organize in HIV than in MoMLV.
It should be noted that in this study we used a recombinant HIV-1 pseudotyped with VSV-G. The mechanisms of recombinant virus entry into cells are therefore different from those for the wild-type virus, mediated by gp120 and CD4 coreceptor interaction. In agreement with previous studies, HIV RTC pseudotyped with VSV-G could be extracted in the absence of detergents (1), thus allowing study of its structure and protein composition in more physiological conditions. In future studies, it will be interesting to investigate which one of the different RTC species described here is competent for nuclear entry and integration.
ACKNOWLEDGMENTS
We are grateful to Bridget Ferns, Dag Helland, and Johnson Mak for antibodies and to Eran Bacharach, Paul Clapham, Guanxia Gao, and Masha Orlova for advice and helpful discussions. We thank Robin Weiss and Yasuhiro Takeuchi for critically reading the manuscript.
A.F. is a Wellcome Trust International Prize Research Fellow. S.P.G. is an Investigator of the Howard Hughes Medical Institute.
REFERENCES
- 1.Bukrinskaya A, Brichacek B, Mann A, Stevenson M. Establishment of a functional human immunodeficiency virus type 1 (HIV-1) reverse transcription complex involves the cytoskeleton. J Exp Med. 1998;188:2113–2125. doi: 10.1084/jem.188.11.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bukrinsky M I, Sharova N, Dempsey M P, Stanwick T L, Bukrinkaya A G, Haggerty S, Stevenson M. Active nuclear import of human immunodeficiency virus type 1 preintegration complexes. Proc Natl Acad Sci USA. 1992;89:6580–6584. doi: 10.1073/pnas.89.14.6580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bukrinsky M I, Haggerty S, Dempsey M I, Sharova N, Adzhubel A, Spitz L, Lewis P, Goldfarb D, Emerman M, Stevenson M. A nuclear localization signal within HIV-1 matrix protein that governs infection of non-dividing cells. Nature. 1993;365:666–669. doi: 10.1038/365666a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bukrinsky M I, Sharova N, McDonald T L, Pushkarskaya T, Tarpley W G, Stevenson M. Association of integrase, matrix and reverse transcriptase antigens of human immunodeficiency virus type 1 with viral nucleic acids following acute infection. Proc Natl Acad Sci USA. 1993;90:6125–6129. doi: 10.1073/pnas.90.13.6125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farnet C M, Bushman F D. HIV-1 cDNA integration: requirement of HMG I(Y) protein for function of preintegration complexes in vitro. Cell. 1997;88:483–492. doi: 10.1016/s0092-8674(00)81888-7. [DOI] [PubMed] [Google Scholar]
- 6.Farnet C M, Haseltine W A. Determination of viral proteins present in the human immunodeficiency virus type 1 preintegration complex. J Virol. 1991;65:1910–1915. doi: 10.1128/jvi.65.4.1910-1915.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fassati A, Goff S P. Characterization of intracellular reverse transcription complexes of Moloney murine leukemia virus. J Virol. 1999;73:8919–8925. doi: 10.1128/jvi.73.11.8919-8925.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferns R B, Tedder R S, Weiss R A. Characterization of monoclonal antibodies against the human immunodeficiency virus (HIV) gag products and their use in monitoring HIV isolate variation. J Gen Virol. 1987;68:1543–1551. doi: 10.1099/0022-1317-68-6-1543. [DOI] [PubMed] [Google Scholar]
- 9.Fouchier R A, Meyer B E, Simon J H, Fischer U, Malim M. HIV-1 infection of non-dividing cells: evidence that the N-terminal basic region of the viral matrix protein is important for Gag processing but not for post-entry nuclear import. EMBO J. 1997;16:4531–4539. doi: 10.1093/emboj/16.15.4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fouchier R A, Meyer B E, Simon J H M, Fischer U, Albright A V, Gonzalez-Scarano F, Malim M H. Interaction of the human immunodeficiency virus type 1 Vpr protein with the nuclear pore complex. J Virol. 1998;72:6004–6013. doi: 10.1128/jvi.72.7.6004-6013.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freed E O, Englund G, Martin M. Role of the basic domain of human immunodeficiency virus type 1 matrix in macrophage infection. J Virol. 1995;72:3949–3954. doi: 10.1128/jvi.69.6.3949-3954.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gallay P, Swingler S, Song J, Bushman F, Trono D. HIV nuclear import is governed by the phosphotyrosine-mediated binding of matrix to the core domain of integrase. Cell. 1995;83:569–576. doi: 10.1016/0092-8674(95)90097-7. [DOI] [PubMed] [Google Scholar]
- 13.Gallay P, Hope T, Chin D, Trono D. HIV-1 infection of nondividing cells through the recognition of integrase by the importin/karyopherin pathway. Proc Natl Acad Sci USA. 1997;94:9825–9830. doi: 10.1073/pnas.94.18.9825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goff S P, Traktman P, Baltimore D. Isolation and properties of Moloney murine leukemia virus mutants: use of a rapid assay for release of virion reverse transcriptase. J Virol. 1981;38:239–248. doi: 10.1128/jvi.38.1.239-248.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grewe C, Beck A, Gelderblom H R. HIV: early virus-cell interactions. J Acquir Immune Defic Syndr. 1990;3:965–974. [PubMed] [Google Scholar]
- 16.Heinzinger N K, Bukrinsky M I, Haggerty S A, Ragland A M, Kewalramani V, Lee A, Gendelman H E, Ratner L, Stevenson M, Emerman M. The Vpr protein of human immunodeficiency virus type 1 influences nuclear localisation of viral nucleic acids in non-dividing host cells. Proc Natl Acad Sci USA. 1994;91:7311–7315. doi: 10.1073/pnas.91.15.7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jenkins Y, McEntee M, Weis K, Greene W. Characterization of HIV-1 Vpr nuclear import: analysis of signals and pathways. J Cell Biol. 1998;143:875–885. doi: 10.1083/jcb.143.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karageorgos L, Li P, Burrell C. Characterization of HIV replication complexes early after cell-to-cell infection. AIDs Res Hum Retroviruses. 1993;9:817–823. doi: 10.1089/aid.1993.9.817. [DOI] [PubMed] [Google Scholar]
- 19.Lee M S, Craigie R. A previously unidentified host protein protects retroviral DNA from autointegration. Proc Natl Acad Sci USA. 1998;95:1528–1533. doi: 10.1073/pnas.95.4.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lewis P, Hensel M, Emerman M. Human immunodeficiency virus infection of cells arrested in the cell cycle. EMBO J. 1992;11:3053–3058. doi: 10.1002/j.1460-2075.1992.tb05376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lewis P, Emerman M. Passage through mitosis is required for oncoretroviruses but not for the human immunodeficiency virus. J Virol. 1994;68:510–516. doi: 10.1128/jvi.68.1.510-516.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McEwen C R. Tables for estimating sedimentation through linear concentration gradients of sucrose solution. Anal Biochem. 1967;20:114–149. doi: 10.1016/0003-2697(67)90271-0. [DOI] [PubMed] [Google Scholar]
- 23.Miller M D, Farnet C M, Bushman F D. Human immunodeficiency virus type 1 preintegration complexes: studies of organization and composition. J Virol. 1997;71:5382–5390. doi: 10.1128/jvi.71.7.5382-5390.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muesing M A, Smith D H, Cabradilla C D, Benton C V, Lasky L A, Capon D J. Nucleic acid structure and expression of the human AIDS/lymphoadenopathy retrovirus. Nature. 1985;313:450–458. doi: 10.1038/313450a0. [DOI] [PubMed] [Google Scholar]
- 25.Naldini L, Blomer U, Gallay P, Ory D, Mulligan R, Gage F H, Verma I M, Trono D. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 26.Popov S, Rexach M, Ratner L, Blobel G, Bukrinsky M. Viral protein R regulates docking of the HIV-1 preintegration complex to the nuclear pore complex. J Biol Chem. 1998;273:13347–13352. doi: 10.1074/jbc.273.21.13347. [DOI] [PubMed] [Google Scholar]
- 27.Risco C, Menendez-Arias L, Copeland T D, Pinto da Silva P, Oroszlan S. Intracellular transport of the murine leukemia virus during acute infection of NIH 3T3 cells: nuclear import of nucleocapsid protein and integrase. J Cell Sci. 1995;108:3039–3050. doi: 10.1242/jcs.108.9.3039. [DOI] [PubMed] [Google Scholar]
- 28.Roe T-Y, Reynolds T C, Yu G, Brown P O. Integration of murine leukemia virus DNA depends on mitosis. EMBO J. 1993;12:2099–2108. doi: 10.1002/j.1460-2075.1993.tb05858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Szilvay A M, Nornes S, Olsen I R, Prasad V R, Endresen C, Goff S P, Helland D E. Epitope mapping of HIV-1 reverse transcriptase with monoclonal antibodies that inhibit polymerase and RNAse H activities. J Acquir Immune Defic Syndr. 1992;5:647–657. [PubMed] [Google Scholar]
- 30.Telesnitsky A, Goff S P. Reverse transcriptase and the generation of retroviral DNA. In: Coffin J M, Hughes S H, Varmus H E, editors. Retroviruses. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1997. pp. 121–160. [PubMed] [Google Scholar]
- 31.Telesnitsky A, Blain S, Goff S P. Assays for retroviral reverse transcriptase. Methods Enzymol. 1993;262:347–362. doi: 10.1016/0076-6879(95)62029-x. [DOI] [PubMed] [Google Scholar]
- 32.Vodicka M A, Koepp D M, Silver P A, Emerman M. HIV-1 Vpr interacts with the nuclear transport pathway to promote macrophage infection. Genes Dev. 1998;12:175–185. doi: 10.1101/gad.12.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wei S Q, Mizuuchi K, Craigie R. A large nucleoprotein assembly at the ends of the viral DNA mediates retroviral DNA integration. EMBO J. 1997;16:7511–7520. doi: 10.1093/emboj/16.24.7511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wei S Q, Mizuuchi K, Craigie R. Footprints on the viral DNA ends in Moloney murine leukemia virus preintegration complexes reflect a specific association with integrase. Proc Natl Acad Sci USA. 1998;95:10535–10540. doi: 10.1073/pnas.95.18.10535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zennou V, Petit C, Guetard D, Nerhbass U, Montagnier L, Charneau P. HIV-1 genome nuclear import is mediated by a central DNA flap. Cell. 2000;101:173–185. doi: 10.1016/S0092-8674(00)80828-4. [DOI] [PubMed] [Google Scholar]