Abstract
The γ134.5 protein of herpes simplex virus (HSV) type 1 functions to prevent the shutoff of protein synthesis mediated by the double-stranded-RNA-dependent protein kinase PKR. This is because γ134.5 associates with protein phosphatase 1 (PP1) through its carboxyl terminus, forming a high-molecular-weight complex that dephosphorylates the α subunit of translation initiation factor eIF-2 (eIF-2α). Here we show that Val193Glu and Phe195Leu substitutions in the PP1 signature motif of the γ134.5 protein abolished its ability to redirect PP1 to dephosphorylate eIF-2α and replication of mutant viruses was severely impaired. The γ134.5 protein, when expressed in Sf9 cells using a recombinant baculovirus, was capable of directing specific eIF-2α dephosphorylation. Deletions of amino acids 258 to 263 had no effect on activity of γ134.5. However, deletions of amino acids 238 to 258 abolished eIF-2α phosphatase activity but not PP1 binding activity. Interestingly, deletions in the AlaArg motif of the carboxyl terminus disrupted the high-molecular-weight complex that is required for dephosphorylation of eIF-2α. These results demonstrate that γ134.5 is functionally active in the absence of any other HSV proteins. In addition to a PP1 binding domain, the carboxyl terminus of γ134.5 contains an effector domain that is required to form a functional complex.
The cellular response to virus infection is a complex process involving different components. The double-stranded-RNA-dependent protein kinase (PKR) is one of the components that play a critical role in antiviral defense (16). In mammalian cells, PKR is induced by interferon, and it is activated by double-stranded RNA. Upon viral infection, PKR is activated to phosphorylate serine 51 on the α subunit of translation initiation factor eIF-2 (eIF-2α). Phosphorylation of eIF-2α increases its affinity for guanine nucleotide exchange factor eIF-2B, thus sequestering eIF-2B complex in an inactive complex with phosphorylated eIF-2 and GDP (12, 13). As a result, eIF-2B is not available to catalyze nucleotide exchange on nonphosphorylated eIF-2, which leads to inhibition of protein synthesis (23). Because viruses synthesize double-stranded RNA during their replication, many of them have evolved mechanisms to counteract PKR, such as blocking the activation of PKR, preventing the phosphorylation of eIF-2α, or promoting the degradation of PKR (16, 35). In addition to its role in antiviral defense, PKR has also been implicated in cellular functions such as growth regulation (4, 5, 33), differentiation (35), and apoptosis in uninfected cells (27, 36).
Previous studies demonstrated that PKR is activated in cells infected with wild-type or mutant herpes simplex virus type 1 (HSV-1). But only in cells infected with γ134.5 null mutants is eIF-2α phosphorylated (6, 18). Therefore, in cells infected with γ134.5 null mutants, initiation of DNA replication triggers the shutoff of total protein synthesis (6, 8, 9). Subsequent studies demonstrated that when human cells are infected with wild-type virus, the γ134.5 protein binds to protein phosphatase 1 (PP1), forming a high-molecular-weight complex that specifically dephosphorylates eIF-2α and thereby prevents the shutoff of protein synthesis (20, 21). Thus, unlike most viruses studied so far, HSV uses a unique strategy to evade the antiviral action of PKR (16).
The γ134.5 protein of HSV-1 consists of 263 amino acids with a large amino-terminal domain, a linker (or swivel) region containing repeats of three amino acids (AlaThrPro), and a carboxyl-terminal domain (10, 11). The triplet repeats are a constant feature of all strains, but the number of repeats varies from strain to strain (10). The carboxyl terminus of the γ134.5 protein is required to interact with PP1 and prevent translation shutoff during HSV-1 infection (9, 19, 21). A prominent feature of this domain is a 64-amino-acid region containing a PP1-interacting signature motif (Arg/Lys)(Val/Ile)XaaPhe (20), which is also present in a number of proteins that complex with PP1 (3, 14, 24, 37, 38, 40). These complexes are involved in diverse functions such as cell division, gene expression, glycogen metabolism, and neurotransmission.
The carboxyl terminus of the γ134.5 protein is homologous to a set of proteins known as GADD34 in human, hamster, and mouse (25, 30, 31, 41) (Fig. 1). GADD34 belongs to a family of proteins expressed under conditions of DNA damage, growth arrest, differentiation, and apoptosis (25, 41). Overexpression of GADD34 facilitates apoptosis induced by gamma radiation; however, its physiological role remains unknown (2, 25). Interestingly, the carboxyl terminus of GADD34 functionally substitutes for the corresponding domain of the γ134.5 protein within the context of HSV-1 genome (19). A hypothesis derived from these studies is that the conserved carboxyl-terminal domain represents a functional module with a common role in virus infection and cellular processes.
FIG. 1.
Amino acid sequence alignment of the carboxyl-terminal domains of the γ134.5 proteins of HSV-1 (10) and HSV-2 (32) and of GADD34 proteins of human (25), mouse (30), and hamster (41).
In the present study, we further examined the role of the carboxyl-terminal domain of the γ134.5 protein. We demonstrate that activation of eIF-2α phosphatase (PP1) by the γ134.5 protein is essential for replication of HSV-1 in infected cells. We also show that γ134.5 protein functions independently of other HSV proteins and provide evidence that the AlaArg motif in the carboxyl terminus of the γ134.5 protein is required to form a high-molecular-weight complex that dephosphorylates eIF-2α.
MATERIALS AND METHODS
Cells and viruses.
The Vero, HeLa, and SK-N-SH cell lines were obtained from the American Type Culture Collection and propagated in Dulbecco's modified Eagle's medium supplemented with 5% (HeLa and Vero) or 10% (SK-N-SH) fetal bovine serum. Sf9 cells were purchased from GIBCO and grown in serum-free, Sf-900 SFM medium supplied by the manufacturer.
HSV-1(F) is a prototype HSV-1 strain used in these studies (15). In recombinant virus R3616, a 1-kb fragment from the coding region of the γ134.5 gene was deleted (7). In recombinant virus R8321 (20), codons encoding Val193 and Phe195 of the γ134.5 gene were replaced with those encoding Glu and Leu, respectively. In addition, this virus contained a 0.5-kb deletion in the thymidine kinase gene. Recombinant virus H9813 was constructed by cotransfection of viral DNA of R8321 (20) with plasmid pRB4867 on rabbit skin cells to restore the thymidine kinase gene. The recombinant progeny was selected and purified in hypoxanthine-aminopterin-thymidine medium. The construct was verified by hybridization of electrophoretically separated restriction enzyme digests with a 32P-labeled BamHI Q fragment as described previously (19). Preparation of viral stock and titration of infectivity were performed on Vero cells.
Recombinant baculoviruses were constructed as suggested by the manufacturer (GIBCO). Briefly, to construct GF9909, the donor plasmid pGF9907 was transformed into Escherichia coli DH10BAC cells, which contained the bacmid with a mini-attTn7 target site and the helper plasmid. White colonies containing recombinant bacmids were selected on Luria-Bertani plates containing kanamycin (50 μg/ml), gentamicin (7 μg/ml), tetracycline (10 μg/ml), X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside;100 μg/ml) and isopropyl-β-d-thiogalactopyranoside (40 μg/ml). Purified recombinant bacmid DNA was then used to transfect Sf9 cells using CellFectin reagent (GIBCO). Virus was harvested 3 days after transfection. In a similar way, plasmids pBH0010, pBH0011, pGF9908, pFastBacHT-gus, pGF2002, pGF2007, pGF2009, and pGF2011 were used to construct recombinant baculoviruses BH0010, BH0011, GF9910, GF9911, GF2003, GF2022, GF2023, and GF2024. Virus constructs were verified by PCR with primers OGF0019 (ATGCAGGATCCGCTAACCCCTCCCACCCCCCCTCACGCCCC) and OGF0013 (ATGCATCCGGATTACAGGGCCCGGGCACGGGCCTCGGGCCCC), which are specific to the γ134.5 gene. Virus titers were determined by plaque assay on Sf9 cells.
Plasmids.
Plasmid pRB4867 contains a BamHI Q fragment of HSV-1(F) in the BamHI site of pUC9 (19). pRB143 contains a BamHI S fragment of HSV-1(F) in the BamHI site of pBR322 (34). pRB4789 contains a BamHI S fragment from pRB143 (26). pGEX-PKR contains cDNA encoding full-length PKR fused in frame to glutathione S-transferase (GST) (a gift from William G. Bryan). pRB4897 contains a BamHI S fragment in which the codons for V193 and F195 in the γ134.5 gene were mutated to those for E and L, respectively (20). pRB4892 contains the coding domain of GST fused to the entire coding domain of PP1 except for the initiator methionine codon (21). pFastBacHTa and pFastBacHTb are expression vectors for making recombinant baculoviruses (GIBCO). pFastBac-gus contains the DNA encoding β-glucuronidase (GIBCO). pRB3027 contains approximately 100 bp of the 5′ untranslated region, the entire coding region of the γ134.5 gene, and the 3′ untranslated region derived from the BamHI S fragment (19).
To construct pGF9908, an EcoRI-SalI fragment, encoding PP1 from pRB4892, was cloned into the EcoRI and SalI sites of pFastBacHTb. To construct pGF9907, a BamHI-StuI PCR fragment containing the entire coding region of the γ134.5 gene was ligated into the BamHI and EcoRV sites of pBluescript II SK(+), resulting in plasmid pGF9901. An NcoI-HindIII fragment from pGF9901 was then ligated into the NcoI and HindIII sites of pFastBacHTb, resulting in plasmid pGF9907. To construct pGF2002, a BstEII-DraIII fragment from pRB4897 was ligated into the BstEII and DraIII sites of pRB3027, resulting in plasmid pBH9902. A BamHI-StuI fragment from pBH9902 was then cloned into the BamHI and EcoRV sites of the pBluescript II SK(+), producing pGF2001. An NcoI-HindIII fragment from pGF2001 was cloned into the NcoI and HindIII sites of pFastBacHTb, yielding pGF2002. In this plasmid, Val193 and Phe195 in the γ134.5 gene were mutated to Glu and Leu, respectively. Cloning and deletions in the γ134.5 gene were done with PCR using pRB143 as the template. To construct pGF2007, a BstEII-BspEI PCR fragment was amplified with oligoBH9716 (CATGGCCCGCCGCCGCCGCCATCGC) and OGF0013 and ligated into the BstEII and BspEI sites of pGF9901, resulting in plasmid pGF2006. An NcoI-BspEI-Klenow fragment was then ligated into the NcoI and StuI sites of pFastBacHTb, yielding pGF2007, which contains the region of the γ134.5 gene encoding amino acids 1 to 253. To construct pGF2009, a BstEII-BspEI PCR fragment was amplified with oligoBH9716 and OGF0015 (ATGCATCCGGATTAGCCGGCTCCGCGGGCCAGGGCCCGGGCA) and ligated into the BstEII and BamHI sites of pGF9901, resulting in plasmid pGF2008. A NcoI-BspEI-Klenow fragment was then ligated into the NcoI and StuI sites of pFastBacHTb, yielding pGF2009, which contains the region of the γ134.5 gene encoding amino acids 1 to 258. To construct pGF2011, a BstEII-BspEI PCR fragment was amplified with oligoBH9716 and OGF0016 (ATGCATCCGGATTACGCCGGGCCGGCTCCGCGGGCCAGGGCC) and ligated into the BstEII and BspEI sites of pGF9901, resulting in plasmid pGF2010. A NocI-BspEI-Klenow fragment was then ligated into the NcoI and StuI sites of pFastBacHTb, yielding pGF2011, which contains the region of the γ134.5 gene encoding amino acids 1 to 260. To construct pBH0010, a BstEII-BspEI PCR fragment encoding amino acids 28 to 238 was amplified and ligated into the BstEII and BspEI sites of pRB4789, yielding plasmid pBH0002. The primers used were oligoBH9716 and oligoBH0005 (AGTCATCCGGATTAGCGGCGCGCCAGGCGGGCGGCCGAGGC). An NcoI-StuI fragment from pBH0002 was then ligated into the NcoI and StuI sites of pFastBacHTa, resulting in pBH0010, which contains the region of γ134.5 encoding amino acids 1 to 238. To construct pBH0003, a BstEII-BspEI PCR fragment encoding amino acids 28 to 248 was amplified with oligoBH9716 and oligoBH0004 (AGTCATCCGGATTAGGCCTCCGCCACCCGGCGCCGGAACCG). The PCR fragment was ligated into the BstEII and BspEI sites of pRB4789, yielding plasmid pBH0003. A NcoI-StuI fragment from pBH0003 was then ligated into the NcoI and StuI sites of pFastBacHTa, resulting in pBH0011, which contains the region encoding amino acids 1 to 248. For plasmids pBH0010, pBH0011, pGF9907, pGF9908, pGF2002, pGF2007, pGF2009, and pGF2011, a His tag was fused in frame to the initiator methionine codon of the γ134.5 gene.
GST pull-down assay.
GST protein and a GST-PP1 fusion protein were induced by the addition of isopropyl-β-d-thiogalactoside to the medium with E. coli BL21 cells transformed with plasmid pGEX4T-1 or pRB4892, followed by affinity purification of the fusion protein from bacterial lysates on agarose beads conjugated with glutathione. Infected Sf9 cells were harvested, washed with cold phosphate-buffered saline, and lysed in buffer containing 50 mM HEPES (pH 7.6), 150 mM NaCl, 10 mM MgCl2, 1% Triton X-100, 0.5 mM phenylmethylsulfonyl fluoride, and 2 mM benzamidine. After 30 min on ice and centrifugation to remove nuclei, the supernatant was precleared with GST beads and then incubated with GST-PP1 fusion protein-bound beads at 4°C overnight. After three washes, the proteins bound to beads were resuspended in disruption buffer containing 50 mM Tris-HCl (pH 7.0), 5% 2-mercaptoethanol, 2% sodium dodecyl sulfate (SDS), and 2.75% sucrose. Samples were subjected to electrophoresis and processed for immunoblot analysis with anti-His tag antibody (1, 8).
eIF-2α phosphatase assays.
Cells either mock infected or infected with viruses were harvested 15 h (HeLa cells) or 48 h (Sf9 cells) postinfection, rinsed with phosphate-buffered saline, resuspended in lysis buffer containing 10 mM HEPES (pH 7.6), 150 mM NaCl, 10 mM MgCl2, 0.2% Triton X-100, 10% glycerol, 0.5 mM phenylmethylsulfonyl fluoride, and 2 mM benzamidine, placed on ice for 30 min, and subjected to centrifugation to remove nuclei. The supernatant fluids were saved for analysis. eIF-2 was purified from rabbit reticulocytes as previously described (17). GST-PKR fusion protein was expressed and purified from E. coli BL21 cells as described above. To prepare 32P-labeled eIF-2α, eIF-2 was incubated with GST-PKR in buffer containing 20 mM Tris-HCl (pH 7.5), 40 mM KCl, 2.0 mM MgCl2, and 0.17 mM [γ32P]ATP (10 Ci/mmol) for 30 min at 34°C. Aliquots of cell lysates were then incubated with phosphorylated eIF-2α in buffer containing 20 mM Tris-HCl (pH 7.5), 40 mM KCl, 2.0 mM MgCl2, and 0.1 mM EDTA at 34°C for 2 min. The reaction was stopped by adding disruption buffer containing 50 mM Tris-HCl (pH 7.0), 5% 2-mercaptoethanol, 2% SDS, and 2.75% sucrose, followed by electrophoresis on a SDS–12% polyacrylamide gel, transferred onto a nitrocellulose membrane, and subjected to autoradiography (21). In addition, the nitrocellulose membrane was scanned by the PhosphorImage SI system, and the radioactivity of eIF-2α was quantitated using ImageQuant NT software (Molecular Dynamics Inc.).
Gel filtration chromatography.
A Superdex 200 HR 10/30 column (1.0 by 30 cm; Amersham Pharmacia Biotech) was equilibrated with 10 mM Tris-HCl (pH 7.5), 50 mM KCl, 2 mM MgCl2, 1 mM dithiothreitol, and 0.1 mM EDTA and pumped at 0.5 ml/min, using a Amersham Pharmacia Biotech fast protein liquid chromatography system. Samples were injected, 0.5-ml fractions were collected on ice, and the absorbance at 280 nm was monitored (20).
Immunoblotting.
Samples were solubilized in the disruption buffer described above, sonicated, boiled, subjected to electrophoresis on SDS-polyacrylamide gels, transferred to nitrocellulose membranes, blocked with 5% nonfat milk, and reacted with anti-γ134.5 antibody (a gift from Bernard Roizman), anti-His tag antibody (Qiagen Inc.), or anti-eIF-2α antibody (a gift from Robert Schneider). The membranes were then rinsed in phosphate-buffered saline and reacted with either goat anti-rabbit or mouse immunoglobulin conjugated to alkaline phosphatase (Bio-Rad) or donkey anti-rabbit or anti-mouse immunoglobulin conjugated to horseradish peroxidase (Amersham Pharmacia Biotech. Inc.) (8, 19).
RESULTS
Activation of eIF-2α phosphatase by the γ134.5 protein is crucial for replication of HSV-1 in infected cells.
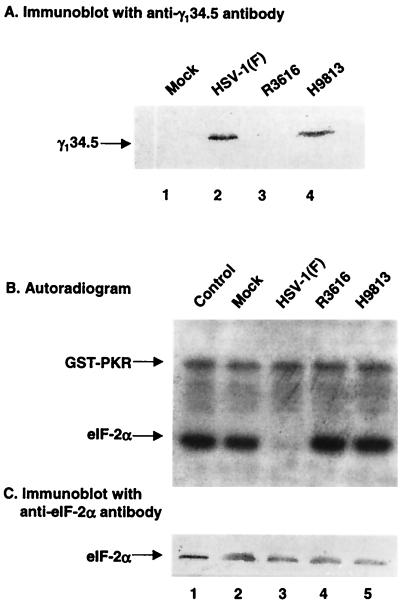
The γ134.5 protein of HSV-1 prevents the total shutoff of protein synthesis in cells infected with HSV-1, which requires an interaction of PP1 with the PP1-interacting motif within the carboxyl terminus of the γ134.5 protein. To determine the contribution of this interaction to viral replication, we constructed a recombinant HSV-1, H9813, by cotransfection of the DNA of R8321 with plasmid pRB4867 to restore the thymidine kinase gene as described in Materials and Methods. In this virus, Val193 and Phe195 in the PP1 signature motif were mutated to Glu and Leu, respectively. The virus construct was verified by restriction digestion and Southern blot analysis (data not shown). Expression of the γ134.5 protein was detected by Western blot analysis with anti-γ134.5 antibody. Mutant H9813 expressed the γ134.5 protein to a level that is similar to that of wild-type HSV-1(F) (see Fig. 3A).
FIG. 3.
(A) Expression of the γ134.5 protein. HeLa cells were mock infected or infected with HSV-1(F), R3616 (in which the coding region of the γ134.5 gene was deleted), or H9813 (in which Vla193Glu and Phe195Leu substitutions were made in the γ134.5 gene) at 10 PFU per cell). At 15 h postinfection, cells were harvested, washed with phosphate-buffered saline, and resuspended in disruption buffer containing 50 mM Tris-HCl (pH 7.0), 5% 2-mercaptoethanol, 2% SDS, and 2.75% sucrose. Samples were then electrophoretically separated on denaturing 12% polyacrylamide gels and transferred to a nitrocellulose membrane. The blot was probed with anti-γ134.5 antibody (1). (B) eIF-2α phosphatase activity in HeLa cells which were mock infected or infected with the indicated viruses. 32P-labeled eIF-2α, prepared as described in Materials and Methods, was incubated with lysates of HeLa cells which were mock infected or infected with indicated viruses at 34°C. After incubation for 2 min, the reaction was stopped by the addition of disruption buffer, and samples were separated electrophoretically on a denaturing 12% polyacrylamide gel, transferred to a nitrocellulose membrane, and subjected to autoradiography (21). Lanes 1, 32P-labeled eIF-2α and GST-PKR not reacted with cell lysates; lanes 2 to 5, 32P-labeled eIF-2α reacted with lysates of cells which were mock infected or infected with the indicated viruses. (C) Immunoblot of the autoradiogram in panel B.
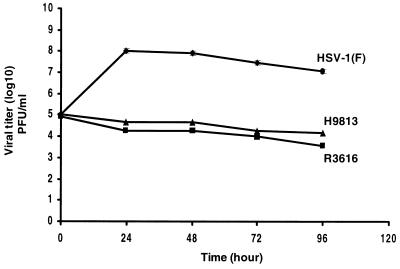
To examine the role of Val193 and Phe195 of the γ134.5 protein in viral replication, confluent human neuroblastoma cells (SK-N-SH) were infected with HSV-1(F), R3616, or H9813 at 0.01 PFU per cell. At different time points postinfection, the cells were harvested and virus yields were determined by plaque assay on Vero cells. As indicated in Fig. 2, in cells infected with HSV-1(F), there was an approximately 3-log increase in viral yield 24 h after infection, reaching a peak titer of 108 PFU/ml. The titer then dropped slightly 96 h postinfection. In contrast, in cells infected with the γ134.5 deletion mutant R3616, the viral yields did not increase after infection and the titer remained approximately 105 PFU/ml. Interestingly, in cells infected with H9813, viral replication resembled that of R3616, in which the γ134.5 gene has been deleted. The results suggest that Val193 and Phe195 in the PP1 interacting motif of the γ134.5 protein are crucial for virus replication.
FIG. 2.
Replication of wild-type HSV-1(F) and the γ134.5 mutants R3616 and H9813 in SK-N-SH cells. Confluent monolayers of cells were infected with viruses at 0.01 PFU per cell and incubated at 37°C. At various times postinfection, the cells were harvested, freeze-thawed three times, and titrated on Vero cells. Duplicate samples were analyzed in parallel at each time point, and the data represent assays from three experiments.
Next, we sought to delineate whether viral replication is linked to eIF-2α phosphatase activity. In this experiment, cell extracts were prepared from HeLa cells either mock infected or infected with HSV-1(F), R3616, or H9813 and tested for their ability to dephosphorylate 32P-labeled eIF-2α. Purified eIF-2 was phosphorylated with GST-PKR and [32P]ATP in vitro and then incubated with cell lysates. As shown in Fig. 3B, the control reaction mixture lacking cell lysate contained two phosphorylated bands, one representing phosphorylated eIF-2α and one representing phosphorylated GST-PKR (lane 1). Incubation of HSV-1(F)-infected cell lysate with the eIF-2–GST-PKR reaction mixture resulted in dephosphorylation of [32P]eIF-2α but not [32P]GST-PKR (Fig. 3B, lane 3). In contrast, cell lysates from cells which were mock infected or infected with R3616 exhibited little or no eIF-2α-specific phosphatase activity (Fig. 3B, lanes 2 and 4). Similarly, H9813-infected cell lysate did not exhibit eIF-2α phosphatase activity (Fig. 3B, lane 5). Western blot analysis with anti-eIF-2α showed that eIF-2α was present in all reaction mixtures (Fig. 3C), confirming that the disappearance of radioactive eIF-2α is due to dephosphorylation. The larger amount of eIF-2α in reaction mixture containing cell lysates is likely due to the presence of endogenous eIF-2α in the lysates (Fig. 3C, lanes 2 to 5). These results demonstrate that Val193 and Phe195 in the PP1 signature motif are critical for the γ134.5 protein to enhance eIF-2α dephosphorylation and that activation of PP1 by the γ134.5 protein is required for viral replication in infected cells.
The activity of the γ134.5 protein is independent of other HSV proteins.
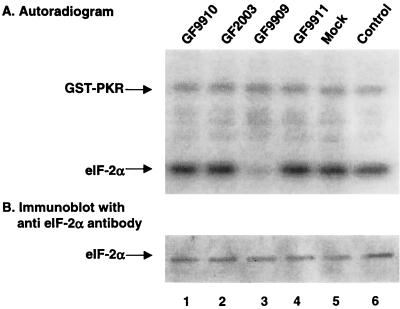
We investigated whether the γ134.5 protein was functionally active in the absence of other HSV proteins. We constructed the following recombinant baculoviruses: GF9909, expressing the wild-type γ134.5 protein; GF2003, expressing the γ134.5 protein with Val193Glu and Phe195Leu mutations in the PP1 binding motif; GF9911, expressing glucuronidase; and GF9910, expressing human PP1. Expression of the γ134.5 protein and PP1 was verified by Western blot with mouse monoclonal anti-His tag antibody, since these proteins were His tagged at the amino terminus (see Fig. 5; also data not shown). To assay for eIF-2α phosphatase activity, monolayers of Sf9 cells were mock infected or infected with GF9909, GF9910, GF9911, or GF2003 at 5 PFU per cell. Forty-eight hours after infection, cells were harvested and cell lysates were incubated with 32P-labeled eIF-2α. Samples were then separated on an SDS–12% polyacrylamide gel, transferred to a nitrocellulose membrane, and subjected to autoradiography. Data in Fig. 4A indicate that lysate of mock infected cells (lane 5) or cells infected with recombinant baculovirus GF9911 expressing glucuronidase (lane 4) did not exhibit eIF-2α phosphatase activity. In contrast, the lysate of cells infected with GF9909 expressing the wild-type γ134.5 protein was able to dephosphorylate eIF-2α (Fig. 4A, lane 3), although it had no effect on phosphorylated GST-PKR. Lysate of cells infected with GF9910 expressing PP1 alone did not display any eIF-2α phosphatase activity. Interestingly, the lysate of cells infected with GF2003 lacked eIF-2α-specific phosphatase activity (Fig. 4A, lane 2). Since GF2003 expresses the γ134.5 protein with Val193Glu and Phe195Leu mutations in the PP1-interacting signature sequence, the result suggests that interaction of the γ134.5 protein with PP1 in Sf9 cells is essential to activate eIF-2α phosphatase. To confirm that there was no degradation of 32P-labeled eIF-2α in the assays, Western blot analysis with anti-eIF-2α antibody (Fig. 4B) indicated that the level of eIF-2α is similar in all samples tested. These results demonstrate that the γ134.5 protein, when expressed in the absence of any other HSV protein, is still capable of redirecting endogenous protein phosphatase in Sf9 cells to dephosphorylate eIF-2α. Importantly, the activity of the wild-type and mutant γ134.5 proteins in Sf9 cells (Fig. 4A) closely reflects the activity seen in HeLa cells infected with HSV-1(F) and H9813 (Fig. 3B).
FIG. 5.
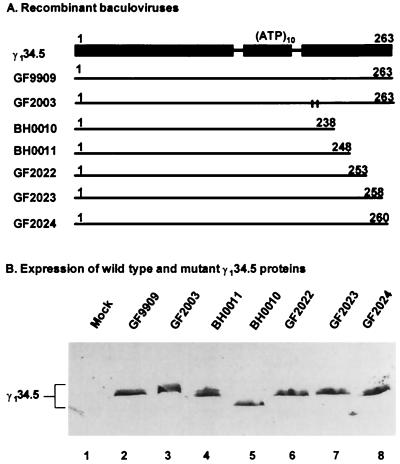
(A) Schematic diagram of recombinant baculoviruses expressing wild-type and mutant forms of the γ134.5 protein. Line 1, domain structure of the wild-type γ134.5 protein. (ATP)10 represents the triplet repeats of AlaThrPro, which connects the amino-terminal domain and the carboxyl-terminal domain. Numbers on the top denote the amino acid positions. Line 2, wild-type γ134.5 protein. Line 3, mutant γ134.5 protein with Val193Glu and Phe195Leu substitutions. Vertical lines indicate the positions of substitutions. Lines 4 to 8, deletion mutants expressing different-length segments of the γ134.5 protein. Numbers on the top of each line indicate the first and last amino acids contained in each construct. (B) Expression of wild-type and mutants of the γ134.5 protein. Cell lysates of Sf9 cells which were mock infected or infected with the indicated viruses were subjected to electrophoresis on a denaturing 12% polyacrylamide gel, transferred to a nitrocellulose membrane, and reacted with anti-His tag antibody (Qiagen Inc).
FIG. 4.
(A) eIF-2α phosphatase activity in Sf9 cells which were mock infected or infected with recombinant baculoviruses. Sf9 cells (107 cells) were either mock infected or infected at 5 PFU per cell with GF9909, which expresses the wild-type γ134.5 protein, GF2003, which expresses the mutant γ134.5 protein with Val193Glu and Phe195Leu substitutions, GF9910, which expresses PP1, or GF9911, which expresses glucuronidase. At 48 h postinfection, cells were harvested and lysates were prepared as described in Materials and Methods. Aliquots of the lysates were then reacted with 32P-labeled eIF-2 at 34°C. After a 2-min incubation, the reaction was stopped by adding disruption buffer, and samples were processed as described for Fig. 3B. Lane 6, 32P-labeled eIF-2α and GST-PKR not reacted with cell lysates; lanes 1 to 5, 32P-labeled eIF-2α and GST-PKR reacted with lysates of cells which were mock infected or infected with the indicated recombinant baculoviruses. (B) Immunoblot of the nitrocellulose membrane in panel A.
The ArgAla motif in the carboxyl terminus is critical for the function of the γ134.5 protein.
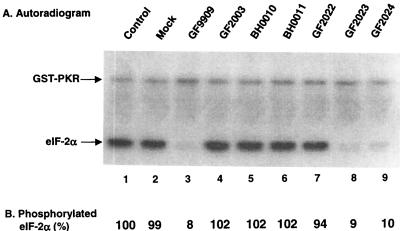
Since the γ134.5 protein modulates eIF-2α phosphatase activity in Sf9 cells, we used this system to investigate the role of the carboxyl terminus of the γ134.5 protein by constructing a series of deletion mutants. Recombinant baculoviruses were constructed to express mutant forms of the γ134.5 protein with deletions from amino acids 238 to 263 (BH0010), 248 to 263 (BH0011), 253 to 263 (GF2022), 258 to 263 (GF2023), or 260 to 263 (GF2024) (Fig. 5A). These mutants, along with GF9909, expressing wild-type γ134.5 protein, and GF2003, expressing the γ134.5 protein with Val193Glu and Phe195Leu mutations in the PP1 signature motif, were used to infect Sf9 cells at 5 PFU per cell. Forty-eight hours after infection, cell lysates were prepared, subjected to electrophoresis on an SDS–12% polyacrylamide gel electrophoresis (PAGE) gel, and processed for immunoblot analysis with mouse monoclonal anti-His tag antibody. As shown in Fig. 5B, these mutants showed protein bands of the expected size and expressed the γ134.5 protein at a level comparable to that of GF9909 expressing wild-type γ134.5 protein.
We next tested the ability of these mutants to activate eIF-2α phosphatase in Sf9 cells. Aliquots of lysate from cells which were mock infected or infected with the γ134.5 expressing viruses were reacted with 32P-labeled eIF2 and samples were processed for autoradiography as described above. As shown in Fig. 6, lysate of cells which were mock infected or infected with GF2003 showed no eIF-2α phosphatase activity (Fig. 6A, lanes 2 and 4). Lysates of cells infected with GF2023 or GF2024 displayed eIF-2α phosphatase activity comparable to that of wild-type γ134.5 protein (Fig. 6A, lanes 3, 8, and 9), indicating that deletion of the last five amino acids in the carboxyl terminus had no effect on the function of the γ134.5 protein. However, lysates of cells infected with GF2022, BH0010, or BH0011 failed to dephosphorylate eIF-2α. PhosphorImager analysis indicated that detectable 32P-labeled eIF-2α was less than 10% after reaction with lysates of cells infected with GF9909, GF2023, and GF2024. In contrast, the level of 32P-labeled eIF-2α remained unchanged for GF2022, BH0010, and BH0011 compared to that in the control reaction mixture (i.e., not reacted with infected cell lysates) (Fig. 6B, lanes 4 to 7 and 1). The data demonstrate that amino acids 238 to 258 of the γ134.5 protein are essential for dephosphorylation of eIF-2α.
FIG. 6.
(A) Activity of the γ134.5 mutants in Sf9 cells. Sf9 cells (107 cells) were mock infected (lane 2) or infected with the indicated recombinant baculoviruses (lanes 3 to 9) at 5 PFU per cells at 27°C. At 48 h after infection, cells were harvested and lysates were prepared as described in Materials and Methods. Aliquots of each lysate were then incubated with 32P-labeled eIF-2α, and samples were processed for autoradiography as described for Fig. 3B. (B) Quantitation of the phosphorylated eIF-2α. Phosphorylated eIF-2α in each lane in panel A was quantitated after eIF-2α phosphatase assays with a PhosphorImage SI system (ImageQuant software). The numbers indicate the percentages of phosphorylated eIF-2α remaining after incubation with the cell lysates relative to that of unreacted eIF-2α.
Deletions of amino acids 238 to 263 from the carboxyl terminus do not affect the association of the γ134.5 protein with PP1.
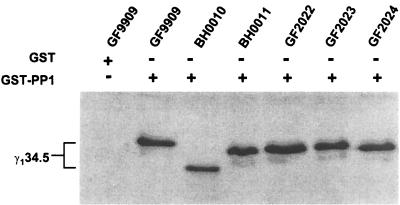
To address whether the deletions of amino acids 238 to 263 in the carboxyl terminus of the γ134.5 protein altered its ability to associate with PP1, we carried out a GST pull-down experiment. GST-PP1 was expressed, purified from E. coli, and incubated with cell extracts prepared from Sf9 cells that were either mock infected or infected with virus at 5 PFU per cell. The protein complexes bound to GST-PP1 were electrophoretically separated on an SDS-PAGE gel and processed for immunoblot analysis with anti-His tag antiserum. As shown in Fig. 7, GST-PP1, but not GST alone, pulled down wild-type γ134.5. In addition, GST-PP1 also pulled down mutant forms of the γ134.5 protein expressed from BH0010, BH0011, GF2022, GF2023, and GF2024. The results indicate that deletions of amino acids 238 to 263 from the carboxyl terminus of the γ134.5 protein do not affect the association of this protein with PP1, as measured under these experimental conditions.
FIG. 7.
Interaction of PP1 with the γ134.5 mutants. Sf9 cells infected with baculovirus expressing wild-type or mutant forms of the γ134.5 protein were harvested at 48 h postinfection and lysed in buffer containing 50 mM HEPES (pH 7.6), 150 mM NaCl, 10 mM MgCl2, and 1% Triton X-100. After 30 min on ice, the lysates were precleared with GST beads and then incubated with GST-PP1 bound to beads at 4°C overnight. After washing three times, the protein complexes were solubilized in disruption buffer, electrophoretically separated on denaturing 12% polyacrylamide gel, and transferred to a nitrocellulose sheet. The blot was probed with anti-His tag antibody (Qiagen Inc).
Deletions in the AlaArg motif of the carboxyl terminus of the γ134.5 protein disrupt the formation of an eIF-2α-specific high-molecular-weight complex.
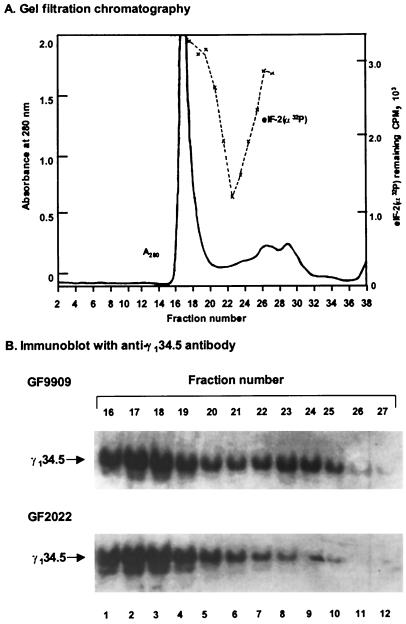
Because binding of the γ134.5 protein to PP1 generates a high-molecular-weight complex that dephosphorylates eIF-2α in HeLa cells infected with HSV-1(F) (20), we investigated whether this complex is formed in Sf9 cells infected with baculoviruses expressing wild-type or mutant forms of the γ134.5 protein. Sf9 cells were first infected with recombinant baculovirus GF9909 expressing the wild-type γ134.5 at 5 PFU per cell, and the cells were harvested 48 h after infection. Lysate was then separated on a Superdex 200 column and fractions were collected. These fractions were assayed for their ability to dephosphorylate 32P-labeled eIF2. The elution profile and eIF-2α phosphatase activity are shown in Fig. 8A. While the bulk of proteins eluted in fractions 15 to 20, eIF-2α phosphatase activity eluted as a single peak in fractions 23 to 24 with a relatively minimum level of protein. Based on calibration of the column with different-sized protein standards, eIF-2α phosphatase activity eluted at a position that corresponds to a molecular weight of 340,000, the same size as the complex containing the γ134.5 protein and PP1 eluted from lysates of HeLa cells infected with HSV-1(F) (20).
FIG. 8.
(A) Superdex 200 gel filtration analysis of cytoplasmic extracts from Sf9 cells infected with GF9909, expressing the wild-type γ134.5 protein. Sf9 cells were infected with 5 PFU of GF9909 per cell at 27°C. At 48 h postinfection, cells were harvested and lysates were prepared and assayed for eIF-2α phosphatase activity as described in Materials and Methods. The dashed line represents 32P-labeled eIF-2α remaining after incubation with aliquots of indicated fractions, measured as described in Materials and Methods. The size of the eIF-2α phosphatase complex (340,000) was estimated with reference to the elution position of the following protein size markers: horse spleen apoferritin (465,000), aldolase (150,000), bovine serum albumin (69,000), ovalbumin (45,000), and rabbit reticulocyte thioredoxin (11,600). (B) Immunoblot of fractions from Superdex 200 column chromatography with anti-γ134.5 antibody. Lysates of Sf9 cells infected with GF9909 or GF2022 were chromatographed as described for panel A. Aliquots of fractions 16 to 27 from each were separated on a SDS–12% polyacrylamide gel, transferred to a nitrocellulose membrane, and reacted with anti-γ134.5 antibody as described in Materials and Methods.
We also analyzed the distribution of the γ134.5 protein in the same Superdex column fractions derived from lysates of cells infected with GF9909. Aliquots of these fractions were subjected to electrophoresis in an SDS–12% PAGE gel and processed for immunoblot analysis with anti-γ134.5 antibody. As shown in Fig. 8B, for the lysate of cells infected with GF9909, a major portion of the γ134.5 protein was in fractions 16 to 19, coincident with a majority of the A280 and representing the void volume of the column. These fractions contain no eIF-2α phosphatase activity (Fig. 8A), and the nature of this γ134.5 peak remains unclear. However, a smaller, second peak of γ134.5 eluted in fractions 22 to 24, which is exactly coincident with the fractions containing eIF-2α phosphatase activity (Fig. 8A and B). We also analyzed lysates of Sf9 cells infected with GF2023 or GF2024 by chromatography on Superdex 200 and observed the same distribution of the γ134.5 protein as was observed from lysate of cells infected with GF9909 (data not shown). These results demonstrate that the γ134.5 protein is a component of a high-molecular-weight complex in Sf9 cells that is capable of dephosphorylating eIF-2α.
Since deletions of amino acids 238 to 258 in the carboxyl terminus of the γ134.5 protein abolished eIF-2α phosphatase activity (Fig. 5 and 6A), we next evaluated whether these deletions had any effect on the formation of a high-molecular-weight complex in Sf9 cells. Lysate of Sf9 cells infected with GF2022, which fails to activate eIF-2α phosphatase (Fig. 6), was chromatographed on the Superdex 200 column, and the distribution of the γ134.5 protein in column fractions was determined as described above. The elution profile is similar to that of GF9909 (data not shown). Results in Fig. 8B indicate that the complete absence of a γ134.5-containing peak eluting in fractions 22 to 24 that corresponds to activated eIF-2α phosphatase (Fig. 8A and B). In contrast, the large peak of γ134.5 not associated with eIF-2α phosphatase in fractions 16 to 19 was not diminished. When lysates of cells infected with BH0010 or BH0011, which also failed to undergo activation of eIF-2α phosphatase, were chromatographed on Superdex 200 and similarly analyzed, each also showed the complete absence of a γ134.5-containing complex in fractions 22 to 24 (data not shown). Collectively, these data indicate that deletions of amino acids 238 to 258 in the γ134.5 protein, notably deletions in the AlaArg motif, disrupted the formation of a high-molecular-weight complex that dephosphorylates the eIF-2α.
DISCUSSION
Several lines of evidence indicate that the γ134.5 protein of HSV-1 is crucial in counteracting the antiviral effect of PKR (6, 8, 9, 20, 21, 28, 29). HSV mutants that fail to express the γ134.5 protein induce a premature shutoff of protein synthesis in cell culture and are highly attenuated in experimental animal models (7, 39). Recent studies demonstrated that the γ134.5 deletion mutants replicate efficiently in PKR−/− knockout mice but not in PKR+/+ mice (28, 29). We have recently found that in HSV-infected cells the γ134.5 protein binds to PP1, forming a high-molecular-weight complex that specifically dephosphorylates eIF-2α (20, 21), and that a PP1-interacting signature motif, (Arg/Lys)(Val/Ile)XaaPhe, in the carboxyl terminus of the protein is required to prevent shutoff of protein synthesis (20). These observations indicate that interaction between the γ134.5 protein and PP1 is critical for down-regulation of PKR activity.
To extend these studies, we further examined the role of the PP1-interacting motif in viral replication. We constructed recombinant virus H9813 and tested its ability to replicate in cell cultures. As shown in Fig. 2, Val193Glu and Phe195Leu substitutions in γ134.5 severely impaired replication of H9813 in SK-N-SH cells. Similar results were seen in mouse fibroblast line T101/2 (data not shown). Moreover, lysate of cells infected with H9813 did not exhibit eIF-2α-specific phosphatase activity. These observations strongly support the view that eIF-2α dephosphorylation mediated by the γ134.5 protein is essential for HSV-1 replication in infected cells. A linkage between viral replication, Val193/Leu195 in the γ134.5 protein, and eIF-2α phosphatase activity underscores the importance of the PP1-interacting signature motif in HSV-1 infection. A number of cellular PP1 binding proteins have been reported to possess the signature sequence (Arg/Lys)(Val/Ile)XaaPhe (3, 14, 22, 24, 38). Among them are DARP-32 (dopamine- and cyclic AMP-regulated phosphoprotein) (40), NIPP1 (nuclear inhibitor of PP1) (38), G subunit (37), and splicing factor PSF (24). The mechanism of PP1 regulation by these proteins remains unclear. It is generally believed that these regulatory proteins either target PP1 to a particular subcellular location or modulate the PP1 catalytic activity towards a specific substrate.
An important finding emerged from our studies is that the γ134.5 protein activates eIF-2α phosphatase activity independent of any other HSV proteins. Significantly, the baculovirus system reproduces observation obtained from HeLa cells infected with HSV-1 (Fig. 3B). As shown in Fig. 4, recombinant baculovirus expressing the wild-type γ134.5 protein is capable of activating eIF-2α phosphatase in Sf9 cells. In addition, gel filtration analysis indicates that the γ134.5 protein is a component of a 340,000-molecular-weight complex that dephosphorylates eIF-2α in Sf9 cells (Fig. 8 and 9). These results parallel the previous findings obtained from HeLa cells infected with HSV-1(F) (20) and further demonstrate that the only HSV protein required for the formation of the high-molecular-weight complex is the γ134.5 protein. These studies also suggest that the γ134.5 protein is likely to interact with PP1 in Sf9 cells, since baculovirus expressing mutant γ134.5 protein, with Val193Glu and Phe195Leu substitutions in the PP1 signature motif, is inactive in Sf9 cells (Fig. 4, lane 2). The implications of these observations are twofold: first, the baculovirus system can be employed to evaluate domain functions of the γ134.5 protein efficiently. Second, it can be used as an alternative system to examine the molecular nature of the γ134.5-PP1 complex. Due to a low level of expression of the γ134.5 protein in HSV-infected cells, it has been difficult to obtain a sufficient amount of the γ134.5-PP1 complex (unpublished data). The use of the baculovirus system will help to resolve this problem.
Our studies support the notion that the carboxyl terminus of the γ134.5 protein consists of a PP1 binding domain and an effector domain. Data in Fig. 6 showed that deletions from amino acids 258 to 263 did not have any effect on the γ134.5 protein activity. Deletions from amino acids 238 to 258 in the carboxyl terminus, however, are deleterious. Although these mutants retain their ability to associate with PP1 (Fig. 7), they were unable to activate eIF-2α phosphatase (Fig. 6), indicating that besides a PP1 interacting domain, the carboxyl terminus of γ134.5 contains an effector domain. It is obvious that communication between the PP1-interacting motif and the extreme carboxyl terminus of γ134.5 determines eIF-2α dephosphorylation. These results defined a region containing amino acids 238 to 258, where a contiguous block of conserved amino acids is found not only in the γ134.5 proteins from HSV-1 and HSV-2 but also in GADD34 from mouse, hamster, and human (Fig. 1). While the roles of these amino acids remain to be elucidated, their involvement in the effector function of γ134.5 is intriguing. It is conceivable that they play similar roles in GADD34 under conditions of DNA damage, growth arrest, and differentiation (25, 30, 31, 41).
Of particular interest is the defective mutant GF2022 with a deletion of 10 amino acids from 253 to 263. This region contains a copy of the AlaArg motif (Fig. 1). Because amino acids 258 to 263 are dispensable (Fig. 5A and 6A), it is most likely that a deletion in AlaArg motif accounted for loss of the function. Although the exact role of AlaArg remains unknown, gel filtration analysis suggests that deletions in the AlaArg motif disrupted the formation of the active γ134.5-PP1 complex (Fig. 8B) and this is responsible for the failure of eIF2α phosphatase to become activated. These data do not eliminate the possibility that the AlaArg repeat may serve as a structural element in the γ134.5 protein. However, the fact that deletions in this motif did not affect the ability of γ134.5 to bind PP1 (Fig. 7) suggests that overall protein structure may not have been changed. It is possible that the AlaArg motif is involved in oligomerization of the γ134.5 protein and PP1. Alternatively, the AlaArg motif may be required for interaction with otherwise unknown components present in the γ134.5-PP1 complex. Work is in progress to address these possibilities.
ACKNOWLEDGMENTS
We thank Bernard Roizman for HSV-1(F), R3616, and anti-γ134.5 antibody, Robert Schneider for anti-eIF-2α antibody, William G. Bryan for plasmid pGEX-PKR, and William Walden and Bellur Prabhakar for critical reading of the manuscript. We are grateful to Suzanne Hessefort for technical assistance.
This work was supported by grant AI 46665 (B.H.) from the National Institute of Allergy and Infectious Diseases.
REFERENCES
- 1.Ackermann M, Chou J, Sarmiento M, Lerner R A, Roizman B. Identification by antibody to a synthetic peptide of a protein specified by a diploid gene located in the terminal repeats of the L component of herpes simplex virus genome. J Virol. 1986;58:843–850. doi: 10.1128/jvi.58.3.843-850.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adler H T, Chinery R, Wu D Y, Kussick S J, Payne J M, Fornace A J, Jr, Tkachuk D C. Leukemic HRX fusion proteins inhibit GADD34-induced apoptosis and associate with the GADD34 and hSNF5/INI1 proteins. Mol Cell Biol. 1999;19:7050–7060. doi: 10.1128/mcb.19.10.7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aitken A, Cohen P. Isolation and characterisation of active fragments of protein phosphatase inhibitor-1 from rabbit skeletal muscle. FEBS Lett. 1982;147:54–58. doi: 10.1016/0014-5793(82)81010-7. [DOI] [PubMed] [Google Scholar]
- 4.Barber G N, Wambach M, Thompson S, Jagus R, Katze M G. Mutants of the RNA-dependent protein kinase (PKR) lacking double-stranded RNA binding domain I can act as transdominant inhibitors and induce malignant transformation. Mol Cell Biol. 1995;15:3138–3146. doi: 10.1128/mcb.15.6.3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chong K L, Feng L, Schappert K, Meurs E, Donahue T F, Friesen J D, Hovanessian A G, Williams B R. Human p68 kinase exhibits growth suppression in yeast and homology to the translational regulator GCN2. EMBO J. 1992;11:1553–1562. doi: 10.1002/j.1460-2075.1992.tb05200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chou J, Chen J J, Gross M, Roizman B. Association of a M(r) 90,000 phosphoprotein with protein kinase PKR in cells exhibiting enhanced phosphorylation of translation initiation factor eIF-2α and premature shutoff of protein synthesis after infection with γ134.5− mutants of herpes simplex virus 1. Proc Natl Acad Sci USA. 1995;92:10516–10520. doi: 10.1073/pnas.92.23.10516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chou J, Kern E R, Whitley R J, Roizman B. Mapping of herpes simplex virus-1 neurovirulence to γ134.5, a gene nonessential for growth in culture. Science. 1990;250:1262–1266. doi: 10.1126/science.2173860. [DOI] [PubMed] [Google Scholar]
- 8.Chou J, Roizman B. The γ134.5 gene of herpes simplex virus 1 precludes neuroblastoma cells from triggering total shutoff of protein synthesis characteristic of programmed cell death in neuronal cells. Proc Natl Acad Sci USA. 1992;89:3266–3270. doi: 10.1073/pnas.89.8.3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chou J, Roizman B. Herpes simplex virus 1 γ134.5 gene function, which blocks the host response to infection, maps in the homologous domain of the genes expressed during growth arrest and DNA damage. Proc Natl Acad Sci USA. 1994;91:5247–5251. doi: 10.1073/pnas.91.12.5247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chou J, Roizman B. The herpes simplex virus 1 gene for ICP34.5, which maps in inverted repeats, is conserved in several limited-passage isolates but not in strain 17syn+ J Virol. 1990;64:1014–1020. doi: 10.1128/jvi.64.3.1014-1020.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chou J, Roizman B. The terminal a sequence of the herpes simplex virus genome contains the promoter of a gene located in the repeat sequences of the L component. J Virol. 1986;57:629–637. doi: 10.1128/jvi.57.2.629-637.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clemens J M, Laing K G, Jeffrey I W, Schofield A, Sharp T V, Elia A, Matys V, James M C, Tilleray V J. Regulation of the interferon-inducible eIF-2 alpha protein kinase by small RNAs. Biochimie. 1994;76:770–778. doi: 10.1016/0300-9084(94)90081-7. [DOI] [PubMed] [Google Scholar]
- 13.De Haro C, Mendez R, Santoyo J. The eIF-2alpha kinases and the control of protein synthesis. FASEB J. 1996;10:1378–1387. doi: 10.1096/fasebj.10.12.8903508. [DOI] [PubMed] [Google Scholar]
- 14.Egloff P M, Johnson D F, Moorhead G, Cohen P T, Cohen P, Barford D. Structural basis for the recognition of regulatory subunits by the catalytic subunit of protein phosphatase 1. EMBO J. 1997;16:1876–1887. doi: 10.1093/emboj/16.8.1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ejercito P M, Kieff E D, Roizman B. Characterization of herpes simplex virus strains differing in their effects on social behaviour of infected cells. J Gen Virol. 1968;2:357–364. doi: 10.1099/0022-1317-2-3-357. [DOI] [PubMed] [Google Scholar]
- 16.Gale M, Jr, Katze M G. Molecular mechanisms of interferon resistance mediated by viral-directed inhibition of PKR, the interferon-induced protein kinase. Pharmacol Ther. 1998;78:29–46. doi: 10.1016/s0163-7258(97)00165-4. [DOI] [PubMed] [Google Scholar]
- 17.Gross M, Kaplansky D A. Identification of a Mr = 39,000 phosphoprotein in highly purified preparations of rabbit reticulocyte eIF-2 that is distinct from the Mr = 35,000 subunit phosphorylated by the hemin-controlled translational repressor. J Biol Chem. 1980;255:6270–6275. [PubMed] [Google Scholar]
- 18.He B, Chou J, Brandimarti R, Mohr I, Gluzman Y, Roizman B. Suppression of the phenotype of γ134.5− herpes simplex virus 1: failure of activated RNA-dependent protein kinase to shut off protein synthesis is associated with a deletion in the domain of the α47 gene. J Virol. 1997;71:6049–6054. doi: 10.1128/jvi.71.8.6049-6054.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He B, Chou J, Liebermann D A, Hoffman B, Roizman B. The carboxyl terminus of the murine MyD116 gene substitutes for the corresponding domain of the γ134.5 gene of herpes simplex virus to preclude the premature shutoff of total protein synthesis in infected human cells. J Virol. 1996;70:84–90. doi: 10.1128/jvi.70.1.84-90.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He B, Gross M, Roizman B. The γ134.5 protein of herpes simplex virus 1 has the structural and functional attributes of a protein phosphatase 1 regulatory subunit and is present in a high molecular weight complex with the enzyme in infected cells. J Biol Chem. 1998;273:20737–20743. doi: 10.1074/jbc.273.33.20737. [DOI] [PubMed] [Google Scholar]
- 21.He B, Gross M, Roizman B. The γ134.5 protein of herpes simplex virus 1 complexes with protein phosphatase 1alpha to dephosphorylate the alpha subunit of the eukaryotic translation initiation factor 2 and preclude the shutoff of protein synthesis by double-stranded RNA-activated protein kinase. Proc Natl Acad Sci USA. 1997;94:843–848. doi: 10.1073/pnas.94.3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Helps N R, Barker H M, Elledge S J, Cohen P T. Protein phosphatase 1 interacts with p53BP2, a protein which binds to the tumour suppressor p53. FEBS Lett. 1995;377:295–300. doi: 10.1016/0014-5793(95)01347-4. [DOI] [PubMed] [Google Scholar]
- 23.Hershey J W. Translational control in mammalian cells. Annu Rev Biochem. 1991;60:717–755. doi: 10.1146/annurev.bi.60.070191.003441. [DOI] [PubMed] [Google Scholar]
- 24.Hirano K, Erdodi F, Patton J G, Hartshorne D J. Interaction of protein phosphatase type 1 with a splicing factor. FEBS Lett. 1996;389:191–194. doi: 10.1016/0014-5793(96)00577-7. [DOI] [PubMed] [Google Scholar]
- 25.Hollander C M, Zhan Q, Bae I, Fornace A J., Jr Mammalian GADD34, an apoptosis- and DNA damage-inducible gene. J Biol Chem. 1997;272:13731–13737. doi: 10.1074/jbc.272.21.13731. [DOI] [PubMed] [Google Scholar]
- 26.Lagunoff M, Roizman B. Expression of a herpes simplex virus 1 open reading frame antisense to the γ134.5 gene and transcribed by an RNA 3′ coterminal with the unspliced latency-associated transcript. J Virol. 1994;68:6021–6028. doi: 10.1128/jvi.68.9.6021-6028.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee S B, Esteban M. The interferon-induced double stranded RNA-activated protein kinase induced apoptosis. Virology. 1994;199:491–496. doi: 10.1006/viro.1994.1151. [DOI] [PubMed] [Google Scholar]
- 28.Leib D A, Harrison T E, Laslo K M, Machalek M A, Moorman N J, Virgin H W. Interferons regulate the phenotype of wild-type and mutant herpes simplex viruses in vivo. J Exp Med. 1999;189:663–672. doi: 10.1084/jem.189.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leib D A, Machalek M A, Williams B R, Silverman R H, Virgin H W. Specific phenotypic restoration of an attenuated virus by knockout of a host resistance gene. Proc Natl Acad Sci USA. 2000;97:6097–6101. doi: 10.1073/pnas.100415697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lord K A, Hoffman-Liebermann B, Liebermann D A. Sequence of MyD116 cDNA: a novel myeloid differentiation primary response gene induced by IL6. Nucleic Acids Res. 1990;18:2823. doi: 10.1093/nar/18.9.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McGeoch D J, Barnett B C. Neurovirulence factor. Nature. 1991;353:609. doi: 10.1038/353609b0. [DOI] [PubMed] [Google Scholar]
- 32.McGeoch D J, Cunningham C, McIntyre G, Dolan A. Comparative sequence analysis of the long repeat regions and adjoining parts of the long unique regions in the genomes of herpes simplex viruses types 1 and 2. J Gen Virol. 1991;72:3057–3075. doi: 10.1099/0022-1317-72-12-3057. [DOI] [PubMed] [Google Scholar]
- 33.Meurs E F, Galabru J, Barber G N, Katze M G, Hovanessian A G. Tumor suppressor function of the interferon-induced double-stranded RNA-activated protein kinase. Proc Natl Acad Sci USA. 1993;90:232–236. doi: 10.1073/pnas.90.1.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Post L E, Conley A J, Mocarski E S, Roizman B. Cloning of reiterated and nonreiterated herpes simplex virus 1 sequences as BamHI fragments. Proc Natl Acad Sci USA. 1980;77:4201–4205. doi: 10.1073/pnas.77.7.4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Proud C G. PKR: a new name and new roles. Trends Biochem Sci. 1995;20:241–246. doi: 10.1016/s0968-0004(00)89025-8. [DOI] [PubMed] [Google Scholar]
- 36.Srivastava S P, Kumar K U, Kaufman R J. Phosphorylation of eukaryotic translation initiation factor 2 mediates apoptosis in response to activation of the double-stranded RNA-dependent protein kinase. J Biol Chem. 1998;273:2416–2423. doi: 10.1074/jbc.273.4.2416. [DOI] [PubMed] [Google Scholar]
- 37.Tang P M, Bondor J A, Swiderek K M, DePaoli-Roach A A. Molecular cloning and expression of the regulatory (RG1) subunit of the glycogen-associated protein phosphatase. J Biol Chem. 1991;266:15782–15789. [PubMed] [Google Scholar]
- 38.Van Eynde A, Wera S, Beullens M, Torrekens S, Van Leuven F, Stalmans W, Bollen M. Molecular cloning of NIPP-1, a nuclear inhibitor of protein phosphatase-1, reveals homology with polypeptides involved in RNA processing. J Biol Chem. 1995;270:28068–28074. doi: 10.1074/jbc.270.47.28068. [DOI] [PubMed] [Google Scholar]
- 39.Whitley R J, Kern E R, Chatterjee S, Chou J, Roizman B. Replication, establishment of latency, and induced reactivation of herpes simplex virus γ134.5 deletion mutants in rodent models. J Clin Investig. 1993;91:2837–2843. doi: 10.1172/JCI116527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Williams K R, Hemmings H C, Jr, LoPresti M B, Konigsberg W H, Greengard P. DARPP-32, a dopamine- and cyclic AMP-regulated neuronal phosphoprotein. Primary structure and homology with protein phosphatase inhibitor-1. J Biol Chem. 1986;261:1890–1903. [PubMed] [Google Scholar]
- 41.Zhan Q, Lord K A, Alamo I, Jr, Hollander M C, Carrier F, Ron D, Kohn K W, Hoffman B, Liebermann D A, Fornace A J., Jr The gadd and MyD genes define a novel set of mammalian genes encoding acidic proteins that synergistically suppress cell growth. Mol Cell Biol. 1994;14:2361–2371. doi: 10.1128/mcb.14.4.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]