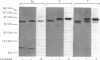
Abstract
The bacterial toxins, choleragen and pertussis toxin, inhibit the light-stimulated GTPase activity of bovine retinal rod outer segments by catalysing the ADP-ribosylation of the alpha-subunit (T alpha) of transducin [Abood, Hurley, Pappone, Bourne & Stryer (1982) J. Biol. Chem. 257, 10540-10543; Van Dop, Yamanaka, Steinberg, Sekura, Manclark, Stryer & Bourne (1984) J. Biol. Chem. 259, 23-26]. Incubation of retinal rod outer segments with NAD+ and a purified NAD+:arginine ADP-ribosyltransferase from turkey erythrocytes resulted in approx. 60% inhibition of GTPase activity. Inhibition was dependent on both enzyme and NAD+, and was potentiated by the non-hydrolysable GTP analogues guanosine 5'-[beta gamma-imido]triphosphate (p[NH]ppG) and guanosine 5'-[beta gamma-methylene]triphosphate (p[CH2]ppG). The transferase ADP-ribosylated both the T alpha and T beta subunits of purified transducin. T alpha (39 kDa), after ADP-ribosylation, migrated as two distinct peptides with molecular masses of 42 kDa and 46 kDa on SDS/polyacrylamide-gel electrophoresis. T beta (36 kDa), after ADP-ribosylation, migrated as a 38 kDa peptide. With purified transducin subunits, it was observed that the GTPase activity of ADP-ribosylated T alpha, reconstituted with unmodified T beta gamma and photolysed rhodopsin, was decreased by 80%; conversely, reconstitution of T alpha with ADP-ribosyl-T beta gamma resulted in only a 19% inhibition of GTPase. Thus ADP-ribosylation of T alpha, the transducin subunit that contains the guanine nucleotide-binding site, has more dramatic effects on GTPase activity than does modification of the critical 'helper subunits' T beta gamma. To elucidate the mechanism of GTPase inhibition by transferase, we studied the effect of ADP-ribosylation on p[NH]pp[3H]G binding to transducin. It was shown previously that modification of transducin by choleragen, which like transferase ADP-ribosylates arginine residues, did not affect guanine nucleotide binding. ADP-ribosylation by the transferase, however, decreased p[NH]pp[3H]G binding, consistent with the hypothesis that choleragen and transferase inhibit GTPase by different mechanisms.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abood M. E., Hurley J. B., Pappone M. C., Bourne H. R., Stryer L. Functional homology between signal-coupling proteins. Cholera toxin inactivates the GTPase activity of transducin. J Biol Chem. 1982 Sep 25;257(18):10540–10543. [PubMed] [Google Scholar]
- Arnone A., Bier C. J., Cotton F. A., Day V. W., Hazen E. E., Jr, Richardson D. C., Yonath A., Richardson J. S. A high resolution structure of an inhibitor complex of the extracellular nuclease of Staphylococcus aureus. I. Experimental procedures and chain tracing. J Biol Chem. 1971 Apr 10;246(7):2302–2316. [PubMed] [Google Scholar]
- Asano T., Katada T., Gilman A. G., Ross E. M. Activation of the inhibitory GTP-binding protein of adenylate cyclase, Gi, by beta-adrenergic receptors in reconstituted phospholipid vesicles. J Biol Chem. 1984 Aug 10;259(15):9351–9354. [PubMed] [Google Scholar]
- Bokoch G. M., Katada T., Northup J. K., Hewlett E. L., Gilman A. G. Identification of the predominant substrate for ADP-ribosylation by islet activating protein. J Biol Chem. 1983 Feb 25;258(4):2072–2075. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bruni P., Hewlett E. L., Moss J. Effects of pertussis toxin-catalyzed ADP-ribosylation on interactions of transducin and the inhibitory GTP-binding protein of adenylate cyclase with guanyl nucleotides. Biochem Biophys Res Commun. 1985 Mar 29;127(3):999–1006. doi: 10.1016/s0006-291x(85)80043-7. [DOI] [PubMed] [Google Scholar]
- Burns D. L., Hewlett E. L., Moss J., Vaughan M. Pertussis toxin inhibits enkephalin stimulation of GTPase of NG108-15 cells. J Biol Chem. 1983 Feb 10;258(3):1435–1438. [PubMed] [Google Scholar]
- Cassel D., Pfeuffer T. Mechanism of cholera toxin action: covalent modification of the guanyl nucleotide-binding protein of the adenylate cyclase system. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2669–2673. doi: 10.1073/pnas.75.6.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassel D., Selinger Z. Catecholamine-stimulated GTPase activity in turkey erythrocyte membranes. Biochim Biophys Acta. 1976 Dec 8;452(2):538–551. doi: 10.1016/0005-2744(76)90206-0. [DOI] [PubMed] [Google Scholar]
- Cassel D., Selinger Z. Mechanism of adenylate cyclase activation by cholera toxin: inhibition of GTP hydrolysis at the regulatory site. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3307–3311. doi: 10.1073/pnas.74.8.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daemen F. J., Riordan J. F. Essential arginyl residues in Escherichia coli alkaline phosphatase. Biochemistry. 1974 Jul 2;13(14):2865–2871. doi: 10.1021/bi00711a014. [DOI] [PubMed] [Google Scholar]
- Foster M., Harrison J. H. Selective chemical modification of arginine residues in mitochondrial malate dehydrogenase. Biochem Biophys Res Commun. 1974 May 7;58(1):263–267. doi: 10.1016/0006-291x(74)90921-8. [DOI] [PubMed] [Google Scholar]
- Fung B. K. Characterization of transducin from bovine retinal rod outer segments. I. Separation and reconstitution of the subunits. J Biol Chem. 1983 Sep 10;258(17):10495–10502. [PubMed] [Google Scholar]
- Fung B. K., Hurley J. B., Stryer L. Flow of information in the light-triggered cyclic nucleotide cascade of vision. Proc Natl Acad Sci U S A. 1981 Jan;78(1):152–156. doi: 10.1073/pnas.78.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill D. M., Meren R. ADP-ribosylation of membrane proteins catalyzed by cholera toxin: basis of the activation of adenylate cyclase. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3050–3054. doi: 10.1073/pnas.75.7.3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. Guanine nucleotide-binding regulatory proteins and dual control of adenylate cyclase. J Clin Invest. 1984 Jan;73(1):1–4. doi: 10.1172/JCI111179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt J. D., Codina J., Risinger R., Birnbaumer L. Identification of a gamma subunit associated with the adenylyl cyclase regulatory proteins Ns and Ni. J Biol Chem. 1984 Feb 25;259(4):2039–2042. [PubMed] [Google Scholar]
- Hong K., Hubbell W. L. Lipid requirements for Rhodopsin regenerability. Biochemistry. 1973 Oct 23;12(22):4517–4523. doi: 10.1021/bi00746a033. [DOI] [PubMed] [Google Scholar]
- Johnson G. L., Kaslow H. R., Bourne H. R. Genetic evidence that cholera toxin substrates are regulatory components of adenylate cyclase. J Biol Chem. 1978 Oct 25;253(20):7120–7123. [PubMed] [Google Scholar]
- Kanaho Y., Tsai S. C., Adamik R., Hewlett E. L., Moss J., Vaughan M. Rhodopsin-enhanced GTPase activity of the inhibitory GTP-binding protein of adenylate cyclase. J Biol Chem. 1984 Jun 25;259(12):7378–7381. [PubMed] [Google Scholar]
- Katada T., Ui M. ADP ribosylation of the specific membrane protein of C6 cells by islet-activating protein associated with modification of adenylate cyclase activity. J Biol Chem. 1982 Jun 25;257(12):7210–7216. [PubMed] [Google Scholar]
- Katada T., Ui M. Direct modification of the membrane adenylate cyclase system by islet-activating protein due to ADP-ribosylation of a membrane protein. Proc Natl Acad Sci U S A. 1982 May;79(10):3129–3133. doi: 10.1073/pnas.79.10.3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koski G., Klee W. A. Opiates inhibit adenylate cyclase by stimulating GTP hydrolysis. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4185–4189. doi: 10.1073/pnas.78.7.4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok-Keung Fung B., Stryer L. Photolyzed rhodopsin catalyzes the exchange of GTP for bound GDP in retinal rod outer segments. Proc Natl Acad Sci U S A. 1980 May;77(5):2500–2504. doi: 10.1073/pnas.77.5.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn H. Light- and GTP-regulated interaction of GTPase and other proteins with bovine photoreceptor membranes. Nature. 1980 Feb 7;283(5747):587–589. doi: 10.1038/283587a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lange L. G., 3rd, Riordan J. F., Vallee B. L. Functional arginyl residues as NADH binding sites of alcohol dehydrogenases. Biochemistry. 1974 Oct 8;13(21):4361–4370. doi: 10.1021/bi00718a019. [DOI] [PubMed] [Google Scholar]
- Manning D. R., Gilman A. G. The regulatory components of adenylate cyclase and transducin. A family of structurally homologous guanine nucleotide-binding proteins. J Biol Chem. 1983 Jun 10;258(11):7059–7063. [PubMed] [Google Scholar]
- Moss J., Stanley S. J., Oppenheimer N. J. Substrate specificity and partial purification of a stereospecific NAD- and guanidine-dependent ADP-ribosyltransferase from avian erythrocytes. J Biol Chem. 1979 Sep 25;254(18):8891–8894. [PubMed] [Google Scholar]
- Moss J., Stanley S. J., Watkins P. A. Isolation and properties of an NAD- and guanidine-dependent ADP-ribosyltransferase from turkey erythrocytes. J Biol Chem. 1980 Jun 25;255(12):5838–5840. [PubMed] [Google Scholar]
- Moss J., Vaughan M. Isolation of an avian erythrocyte protein possessing ADP-ribosyltransferase activity and capable of activating adenylate cyclase. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3621–3624. doi: 10.1073/pnas.75.8.3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss J., Watkins P. A., Stanley S. J., Purnell M. R., Kidwell W. R. Inactivation of glutamine synthetases by an NAD:arginine ADP-ribosyltransferase. J Biol Chem. 1984 Apr 25;259(8):5100–5104. [PubMed] [Google Scholar]
- Navon S. E., Fung B. K. Characterization of transducin from bovine retinal rod outer segments. Mechanism and effects of cholera toxin-catalyzed ADP-ribosylation. J Biol Chem. 1984 May 25;259(10):6686–6693. [PubMed] [Google Scholar]
- Northup J. K., Sternweis P. C., Smigel M. D., Schleifer L. S., Ross E. M., Gilman A. G. Purification of the regulatory component of adenylate cyclase. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6516–6520. doi: 10.1073/pnas.77.11.6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pober J. S., Bitensky M. W. Light-regulated enzymes of vertebrate retinal rods. Adv Cyclic Nucleotide Res. 1979;11:265–301. [PubMed] [Google Scholar]
- Powers S. G., Riordan J. F. Functional arginyl residues as ATP binding sites of glutamine synthetase and carbamyl phosphate synthetase. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2616–2620. doi: 10.1073/pnas.72.7.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K. The reaction of phenylglyoxal with arginine residues in proteins. J Biol Chem. 1968 Dec 10;243(23):6171–6179. [PubMed] [Google Scholar]
- Van Dop C., Tsubokawa M., Bourne H. R., Ramachandran J. Amino acid sequence of retinal transducin at the site ADP-ribosylated by cholera toxin. J Biol Chem. 1984 Jan 25;259(2):696–698. [PubMed] [Google Scholar]
- Van Dop C., Yamanaka G., Steinberg F., Sekura R. D., Manclark C. R., Stryer L., Bourne H. R. ADP-ribosylation of transducin by pertussis toxin blocks the light-stimulated hydrolysis of GTP and cGMP in retinal photoreceptors. J Biol Chem. 1984 Jan 10;259(1):23–26. [PubMed] [Google Scholar]
- Watkins P. A., Moss J., Burns D. L., Hewlett E. L., Vaughan M. Inhibition of bovine rod outer segment GTPase by Bordetella pertussis toxin. J Biol Chem. 1984 Feb 10;259(3):1378–1381. [PubMed] [Google Scholar]
- Watkins P. A., Moss J. Effects of nucleotides on activity of a purified ADP-ribosyltransferase from turkey erythrocytes. Arch Biochem Biophys. 1982 Jun;216(1):74–80. doi: 10.1016/0003-9861(82)90189-8. [DOI] [PubMed] [Google Scholar]
- West R. E., Jr, Moss J., Vaughan M., Liu T., Liu T. Y. Pertussis toxin-catalyzed ADP-ribosylation of transducin. Cysteine 347 is the ADP-ribose acceptor site. J Biol Chem. 1985 Nov 25;260(27):14428–14430. [PubMed] [Google Scholar]