Abstract
Glioblastoma multiforme (GBM) is one of the deadliest human malignancies. A cure for GBM remains elusive, and the overall survival time is less than 1 year. Thus, the development of more efficient therapeutic approaches for the treatment of these patients is required. Induction of tumor cell death by certain phytochemicals derived from medicinal herbs and dietary plants has become a new frontier for cancer therapy research. Although the cancer suppressive effect of Ficus carica (fig) latex (FCL) has been determined in a few cancer types, the effect of this latex on GBM tumors has not been investigated. Therefore, in the current study, the anti-proliferative activity of FCL and the effect of the FCL–temozolomide (TMZ) combination were tested in the T98G, U-138 MG, and U-87 MG GBM cell lines using the WST-1 assay. The mechanism of cell death was analyzed using Annexin-V/FITC and TUNEL assays, and the effect of FCL on invasion was tested using the chick chorioallantoic membrane assay. To determine the effect of FCL on GBM progression, the expression levels of 40 GBM associated miRNAs were analyzed in T98G cells using RT-qPCR. According to the obtained data, FCL causes cell death in GBM cells with different responses to TMZ, and this effect is synergistically increased in combination with TMZ. In addition, the current study is the first to demonstrate the effect of FCL on modulation of let-7d expression, which may be an important underlying mechanism of the anti-invasive effect of this extract.
Keywords: Ficus carica latex, Glioblastoma, Temozolomide, microRNA, Invasion
Introduction
Glioblastoma multiforme (GBM) is the most common malignant brain tumor and is one of the deadliest human malignancies (Krex et al. 2007; Hulleman and Helin 2005). These tumors are highly invasive, rapidly spreading central nervous system cancers and are resistant to surgical and medical treatment. Although advanced therapy protocols are palliative for most patients, a cure remains elusive, and the overall survival time of GBM patients is typically less than 1 year (Stupp et al. 2005; Louis et al. 2007). For this reason, there is an increasing need for the development of more efficient therapeutic approaches for the treatment of these patients. Induction of tumor cell death by certain phytochemicals derived from medicinal herbs and dietary plants has become a new frontier for cancer therapy research (Mijatovic et al. 2011). Most conventional drugs are established natural products or are directly derived from them (Newman and Cragg 2007).
Ficus carica (fig) originated in the Middle East and is currently an important crop worldwide. The common fig still grows wild in the Mediterranean basin (Mawa et al. 2013). Therapeutic usage of fig products is widespread in the Middle East (Conforti et al. 2012). Latex released by picking the fruits has some therapeutic effects (Ahmed et al. 1988). In addition to well-known hypoglycemic (Serraclara et al. 1998), hypocholesterolemic (Canal et al. 2000), hypotriglyceridemic (Pérez et al. 1999), and anthelmintic effects of Ficus carica latex (FCL), its cancer suppressive (Rubnov et al. 2001) effects were investigated in a few recent studies. Basically, these studies involved the following cell lines: Raji and DG75 Burkitt B cell lymphoma, Jurkat and HD-MAR T-cell leukemia, DU-145 prostate cancer, MCF-7 breast cancer (Rubnov et al. 2001), stomach cancer (Hashemi et al. 2011), and A375 melanoma (Menichini et al. 2012). To improve the understanding of the anti-carcinogenic activity of FCL, further studies are required.
One of the mechanisms that are critically involved in the progression of cancer is microRNAs (miRNAs). Alterations in miRNA expression and function contribute to the initiation, maintenance, progression, invasiveness, metastasis, and acquisition of drug resistance in tumors (Di Leva and Croce 2010; Gandellini et al. 2011). Thus, targeting deregulated miRNAs with nontoxic chemopreventive agents could be a promising strategy for cancer therapy (Sethi et al. 2013).
Although GBM is one of the most aggressive tumor types, the effect of FCL on these tumors has not been evaluated. Therefore, the first aim of this study was to analyze the anticancer effects of FCL on GBM cells that differ with respect to their responses to temozolomide (TMZ). Therefore, we analyzed and compared the anticancer effect of FCL in the U-138 MG, T98G, and U-87 MG cell lines. The second aim was to evaluate the combined effect of TMZ and FCL in human GBM cells. The third aim of this study was to determine the differences in GBM progression associated with miRNA expression levels before and after FCL and TMZ treatment.
Materials and Methods
FCL Production
FCL was collected from fig trees in Bursa (Turkey) drop-by-drop by cutting young leaves, and the collected samples were stored at −20 °C. Ultrasound-assisted extraction was performed in a temperature controlled ultrasonic cleaner. Furthermore, the temperature was also monitored with a thermometer. The FCL was placed in a glass vial (45 ml), and hexane solvent (5 ml) (Merck, Darmstadt, Germany) was added; then the vial was placed in an ultrasonic cleaning bath (United) at 40 kHz for 30 min, after which the hexane extract was fractioned from FCL sample. Then the FCL sample was fractionated with 5 ml of dichloromethane and ethanol (Merck, Darmstadt, Germany), respectively, using the ultrasound-assisted extraction method. The ethanol fractionated extract was evaporated to dryness, and ethanolic residue was dissolved in distilled water. The hexane, dichloromethane, ethanol, and water fractions were used for the analysis. The fractionation flow of FCL extract is described in Fig. 1a.
Fig. 1.
a. A schematic diagram depicting the fractionation of FCL extracts. b HPLC chromatogram of water fraction of FCL at 280 nm. Peaks 1, 2, 3, 4, and 5 correspond to protocatechuic acid, caffeic acid, p-coumaric acid, ferulic acid, and quercetin, respectively
HPLC–DAD Analysis
An Agilent 1200 HPLC system (Waldbronn, Germany), consisting of a vacuum degasser, a binary pump, an autosampler, and a diode-array detector, was used for the determination of phenolic compounds in fractions. Chromatographic separations were performed using an XBridge C18 (4.6 × 250 mm, 3.5 µm) column from Waters (Elstree, UK). The mobile phase consisted of 1 % formic acid in water (solvent A) and acetonitrile (solvent B) (Merck, Darmstadt, Germany). The gradient conditions were as follows: 0–10 min 13 % B, 10–20 min 41.5 % B, 20–25 min 70 % B, 25–35 min 10 % B, and a total run time of 35 min. The column was equilibrated for 10 min prior to each analysis at 25 °C. The flow rate was 0.5 ml/min, and the injection volume was 10 µl. A Chemstation for LC (Agilent) was used for data acquisition and pre-processing. The monitoring wavelengths of interest were 280 nm for protocatechuic acid, 320 nm for caffeic acid, ferulic acid, and p-coumaric acid (Merck, Darmstadt, Germany), and 360 nm for quercetin (Sigma–Aldrich, St. Louis, Missouri, USA). Peaks were identified based on a comparison of retention times and UV spectra with standards of protocatechuic acid, caffeic acid, ferulic acid, p-coumaric acid, and quercetin.
Cell Line Maintenance
The T98G, U-138 MG, and U-87 MG human GBM cell lines were provided by the American Type Culture Collection (ATCC; Rockville, USA). The cells were grown in Dulbecco’s Modified Eagle’s Medium-F12 (DMEM-F12; HyClone, Utah, USA) containing l-glutamine supplemented with 10 % fetal bovine serum (FBS, BIOCHROME, Berlin, Germany), 1 mM sodium pyruvate, 100 µg/ml streptomycin, and 100 U/ml penicillin in a humidified 5 % CO2 incubator at 37 °C.
Cytotoxicity and Cell Viability
The cytotoxicity of ten different doses of FCL in the T98G, U-138 MG, and U-87 MG cell lines was assayed using a cell proliferation kit (WST-1, Roche Applied Sciences, Mannheim, Germany) after 24 and 48 h of incubation according to the manufacturer’s instructions. All analyses were performed in quadruplicate. The results were expressed as a percentage of the untreated controls. The absorbance of the untreated control cells was set at 100 %, and the absorbance of FCL-treated cells was measured as the surviving percentage. The following formula was used to calculate the percent inhibition:
Evaluation of the Viability of Human Peripheral Blood Lymphocytes
Human peripheral blood lymphocytes were used for in vitro viability assays. Heparinized total blood (5 ml) was obtained from two healthy, non-smoking volunteers, one male and one female who were 40 and 21 years old, respectively, after obtaining their complete informed consent. Human mononuclear lymphocytes were isolated as described previously (Tunca et al. 2012). Two cultures were prepared from each volunteer’s blood sample. For the viability assay, cells were exposed to 0.25 mg/ml FCL. An untreated culture was used as the negative control, and 30 mM H2O2 treated cells were used as the positive control.
Measurement of the Effect of FCL on Apoptosis
Annexin-V-FITC/PI Analysis
The percentage of apoptotic T98G, U-138 MG, and U87 MG cells was assessed using an Annexin-V-FITC/propidium iodide (PI) binding kit, (BD Pharmagen™ San Jose, CA, USA) using flow cytometry (FACSCanto, Becton–Dickinson, USA) before and after culture with or without the addition of FCL according to the manufacturer’s specifications. Cells that stained only for Annexin V were considered to be in early apoptosis, and those that stained for both Annexin-V and PI were considered to be in late apoptosis or necrosis. Cells that were negative for Annexin-V/PI were considered to be viable. Annexin-V-FITC/PI analysis was duplicated for all cell lines and FCL and/or TMZ treatments.
TUNEL Assay
For the TUNEL assays, the T98G, U-138 MG, and U-87 MG cells were cultured in 4-well chamber slides (Millicell EZ Slide, Millipore, USA). After 24 h of culture with or without the FCL, an ApopTag In Situ Apoptosis Detection Kit (Intergen, Purchase, NY, USA) was used to detect apoptotic cells according to the manufacturer’s instructions. Briefly, the cells were fixed with 1 % paraformaldehyde, permeabilized with 0.3 % Triton X-100 and then washed three times with PBS. DNA breaks were labeled by incubation (1 h, 37 °C) with terminal deoxynucleotidyl transferase and a nucleotide mixture containing fluorescein isothiocyanate-conjugated dUTP. The cell nuclei were stained with PI, and the TUNEL-positive and total nuclei were observed under a fluorescent microscope (Nikon, Japan). More than 1500 nuclei were counted per field, and the experiment was performed twice.
Evaluating the Effect of FCL on Invasion
Chick Chorioallantoic Membrane (CAM) Assay
Fertilized chick eggs were incubated at 37 °C and 60 % humidity until 7 days post-fertilization at which time a window was opened in the egg shell, and 1 million T98G cells were applied to the CAM. Next, 0.25 mg/ml per CAM of FCL was added directly to the tumor cells on the CAM surface. As a negative control, T98G cells alone were applied to the CAM. Tumors were allowed to continue to grow for 10 days, after which the tumor mass was removed and weighed. Both FCL-treated and untreated tumors were harvested 10 days later and homogenized, and VEGF expression levels were measured. A total of 24 eggs comprised the untreated control group, and the FCL experimental analyses were performed on 23 eggs.
VEGF Expression Analyses
To evaluate the expression of the vascular endothelial growth factor (VEGF) gene in response to FCL treatment in the CAM assay, total RNA was extracted using the PureLink TM RNA Mini Kit (Ambion, USA) following the manufacturer’s protocol. RNAs were reverse transcribed using a cDNA Synthesis Kit (New England Biolabs, UK). The samples were then analyzed using RT-qPCR to profile the expression levels of VEGF (NM_003376); we also evaluated the expression level of the human beta actin (ACTB) housekeeping gene. Gene expression analyses were performed in duplicate for each sample. Only samples with Ct values less than 35 were included in the further analyses. PCR was performed in a 20-µl reaction mixture that contained 5 µl of cDNA as a template, 10 µM specific oligonucleotide primer pairs, and SYBR Green qPCR master Mix (Qiagen, Germantown, MD). The cycle parameters were as follows: 95 °C for 10 min, 45 cycles at 95 °C for 15 s, and 60 °C for 60 s, followed by melting curve analysis in the LightCycler 480II (Roche Diagnostics, USA). The absence of genomic DNA contamination was confirmed by performing a no reverse transcription control with RNA samples using an ACTB RT-qPCR primer assay. The initial copy number of the samples and the threshold cycle (Ct) for mRNA expression were determined using the Light Cycler 480II software (Roche Diagnostics, Indianapolis, USA). The 2−ΔCt method was used to calculate the fold change in mRNA expression between the tested samples (Livak and Schmittgen 2001).
Determination of the Combined Effect of FCL and TMZ
The effective doses of TMZ were determined to be 450 µM for the T98G and U-138 MG cell lines and 25 µM for the U87 MG cell line in previous studies (Yoshino et al. 2010). To evaluate the combined effect of FCL and TMZ, cells were seeded at a density of 2 × 104 cells/well in 96-well plates. For the proliferation assays, the cells were exposed to doses of FCL and TMZ which were found to be effective in the T98G, U-138 MG, and U-87 MG cells, the combination of TMZ and FCL, or H2O2 at 24 or 48 h after plating. The dose–response cell viability curves after treatment with FCL alone, TMZ alone, or the combination were analyzed. The nature of the interaction between TMZ and FCL was evaluated using WST-1 analysis. All analyses were performed in quadruplicate for all cell lines.
Evaluation of the Effect of FCL or TMZ on miRNA Expression Profiles of GBM Cells
miRNA expression profiling was performed to evaluate the molecular effect of FCL on T98G cells. Cells were seeded at 3 × 105/well in 6-well plates to analyze the miRNA expression levels. After 24 h of culture in normal growth medium, the cells were exposed to one of the following: 0.25 mg/ml FCL, 450 µM TMZ, a combination of 450 µM TMZ and 0.25 mg/ml FCL, or growth medium as a positive control over a 24-h incubation in a humidified incubator.
Total RNA was extracted after 24 h of incubation using the miRNeasy Mini Kit (QIAGEN, Germantown, Maryland, USA) following the manufacturer’s protocol. Total RNA (5 ng) was reverse transcribed using the RT2 miRNA First Strand Kit (QIAGEN, Germantown, Maryland, USA). Samples were analyzed for the presence and differential expression of 40 miRNAs related to drug resistance and GBM formation using cancer RT2 miRNA PCR arrays (RT2 Profiler; SABiosciences, Frederick Md, USA) according to the manufacturer’s instructions. The accession numbers of the primers are shown in Table 1. The thermal cycling conditions for all assays were as described previously (Tunca et al. 2012). Real time PCR analyses were performed in the LightCycler 480II (Roche Diagnostics, USA). RNA input was normalized to endogenous controls: SNORD 44, SNORD 47, and SNORD 48 for miRNAs and the TATA-binding protein for protein encoding genes. The initial copy number in the samples and the threshold cycle (Ct) for miRNA expression were determined using the Light Cycler 480II’s software (Roche Diagnostics, USA). The average Ct value of up to three housekeeping genes from this assay was used as a baseline to normalize the PCR Array data. The miRNA Reverse Transcription Control Assay was used to test the efficiency of the miScript II Reverse Transcription Kit reaction using a primer set to detect a template synthesized from the kit’s built-in miRNA External RNA Control. Positive PCR control assays were used to test the efficiency of the polymerase chain reaction chemistry as well as the instrument using a predispensed artificial DNA sequence and a primer set designed to detect it. Two independent RT2 miRNA PCR arrays were analyzed for each sample.
The 2(−DCt) method was used to calculate the fold change in miRNA expression between the tested samples (Livak and Schmittgen 2001). Data analysis was performed with a Web-based software package for the miRNA PCR array system (miScript miRNA PCR Array Data Analysis; http://www.sabiosciences.com/pcr/arrayanalysis.php).
Validation of miRNA Expression with RT-PCR Assay
To validate the capacity of FCL to modify miRNA expression in GBM, T98G cells were treated for 24 h with one of the following: 0.25 mg/ml FCL, 450 µM TMZ, a combination of 450 µM TMZ and 0.25 mg/ml FCL, or growth medium as a positive control in 6-well culture plates over a 24-h incubation in a humidified incubator.
Then cells were subjected to total RNA extraction using RNeasy kits (Qiagen, Germantown, MD). All RNA samples were assessed for RNA quantity and quality using the NanoDrop 2000 Spectrophotometer. Protein and chemical contamination was determined by obtaining 260:280 and 260:230 ratios for each RNA sample. RNA samples with 1.8–2.0 for 260:280 ratios, >1.8–260:230 ratios, and with a total concentration ranging from 200 to 400 ng/µl were selected for cDNA synthesis. Total RNA (5 ng) of cells was reverse transcribed using the RT2 miRNA First Strand Kit (Qiagen, Germantown, Maryland, USA). The samples were analyzed for the presence and differential expression of let-7d (MIMAT0000065), which is modulated by FCL in T98G cells according to RT2miRNA PCR arrays (RT2 Profiler; Qiagen, Frederick Md, USA). miRNA expression analyses were performed ten times for each sample. Thermal cycling conditions for all assays were 95 °C for 10 min, 45 cycles at 95 °C for 15 s, and 60 °C for 30 s, followed by melting curve analysis in the Light Cycler 480II (Roche Diagnostics, Indianapolis, USA). RNA input was normalized to endogenous control SNORD 48 for miRNAs and the TATA-binding protein for protein encoding genes. The initial copy number in the samples and the threshold cycle (Ct) for miRNA expression were determined using the Light Cycler 480II software (Roche Diagnostics, Indianapolis, USA). The miRNA Reverse Transcription Control Assay was used to test the efficiency of the miScript II Reverse Transcription Kit reaction using a primer set to detect a template synthesized from the kit’s built-in miRNA External RNA Control. Positive PCR control assays were used to test the efficiency of the polymerase chain reaction chemistry and of the instrument using a predispensed artificial DNA sequence and a primer set designed to detect the sequence. The 2−ΔCt method was used to calculate the fold change in miRNA expression between the tested samples (Livak and Schmittgen 2001).
Statistical Analyses
One-way ANOVA and Tukey’s analyses were used to determine the statistical significance of the WST-1 data using SPSS 16 statistical software. RT2 Profiler™ PCR Array Data Analysis was used to determine the statistical significance of the changes in mRNA and miRNA expression. Values of p < 0.05 were considered to be statistically significant.
Results
Active Compounds in FCL
To determine the active compound of FCL, hexane, dichloromethane, ethanol, and water fractions of the crude extract were fractioned. Because we did not define any cytotoxic activity on the hexane, dichloromethane, and ethanol fractions, we only focused on the active component of water fraction of FCL on HPLC analyses.
Phenolic compounds in FCL were detected using HPLC–DAD. Protocatechuic acid, caffeic acid, ferulic acid, p-coumaric acid, and quercetin were detected in the water fraction of FCL extract. The FCL HPLC chromatogram is shown in Fig. 1b The levels of protocatechuic, caffeic, ferulic, p-coumaric acids, and quercetin were calculated as 28.784 ± 0.080, 3.06 ± 0.027, 2.536 ± 0.005, 2.746 ± 0.007, and 0.562 ± 0.004 mg/L, respectively, in the water fraction of FCL.
FCL Inhibits GBM Cell Proliferation In Vitro
T98G, U-138 MG, and U-87 MG cells were seeded at a density of 2 × 104 cells/well in 96-well plates. Cell proliferation was assessed using the WST-1 assay after 24–72 h of exposure to FCL doses ranging from 0.125 to 2 mg/ml. All three cell lines exhibited reduced cell numbers in a dose- and time-dependent manner (Fig. 2).The inhibitory concentration at which 50 % of the cells died within 24 h was identified (IC50). The percentage decrease in the proliferation of T98G was 58.7 %, in U-138 MG was 54.94 %, and in U-87 MG was 68.81 % at 0.25 mg/ml FCL concentration (Fig. 2). When T98G, U-138 MG, and U87-MG cells were treated with H2O2, we observed 89.67, 68.7, and 87.60 % reduction in proliferation in 24 h, respectively.
Fig. 2.
Antitumor effects of FCL against T98G, U-138 MG and U87 MG cell lines. *(IC50) and p < 0.05; Evaluated using one-way ANOVA and Tukey’s tests using SPSS 16.00 software for Windows (IBM, Chicago, IL).
Low cytotoxic effects were noted when fresh human mononuclear lymphocytes were treated with 0.25 mg/ml FCL. After incubation with FCL, the reduction in proliferation was determined to be 14.83 ± 0.33 % in 24 h, 23.00 ± 2.12 % in 48 h, and 29.75 ± 2.05 % in 72 h compared with untreated cultures, whereas when lymphocytes were treated with H2O2, the reduction in proliferation was 75.5 ± 0.5, 80.00 ± 1.08, and 87.00 ± 1.22 %, respectively.
FCL Stimulated Apoptosis in the T98G, U-138 MG, and U-87 MG Cell Lines
To determine the type of cell death (apoptosis or necrosis) induced by FCL, the cells were subjected to Annexin-V-FITC/PI and TUNEL assays before and after FCL treatment. The T98G, U-138 MG, and U-87 MG cells were treated with 0.25 mg/ml FCL for 24 h. Based on the Annexin V analyses, the percentage of apoptotic cell death in the T98G, U-138 MG, and U-87 MG cells after FCL treatment was 24.7, 32.7 and 58.0 %, respectively (Fig. 3). In the TUNEL assays, the percentages of apoptotic cells observed in the T98G, U-138 MG, and U-87 MG cells treated with 0.125 mg/ml FCL were 24.5, 32.5 and 57.8 %, respectively (Fig. 4).
Fig. 3.
Apoptosis induced by 0.25 mg/ml FCL at 24 h based on the Annexin V-FITC/PI assay.
Fig. 4.
TUNEL assay performed 24 hours after the addition of FCL (x10). The first column shows untreated control cells. The second column shows H2O2-treated (30 mM) positive control cells. The third column shows cells treated with 0.25 mg/ml FCL.
FCL Reduces Invasion in GBM Cells
T98G cells implanted on the surface of the chick CAM grow and stimulated neovascularization. In 13 of the eggs without FCL treatment of the implanted tumor cells, new blood vessels formed during the 10 day study and invaded the tumor mass as it grew. Four of the eggs died, and no tumor growth was observed in seven of these eggs. On the other hand, in 9 of the eggs treated with FCL, neovascularization was greatly reduced, and tumor size was reduced. Two of the eggs died, and there was no tumor growth in 12 of these eggs. Based on the measurements of tumor weight, the average tumor size with untreated tumor cells was 0.013 ± 0.002 gr, and the average tumor size with FCL-treated cells was 0.008 ± 0.001 gr (p = 0.08, Independent sample t test) (Fig. 5).
Fig. 5.
Effect of FCL on new blood vessel growth based on the CAM assay.
To evaluate the effect of FCL on invasion in GBM, VEGF expression was analyzed in tumors grown with untreated cells and in 0.25 mg/ml FCL-treated tumors grown in the CAM model.
According to the RT2 Profiler PCR Array Data Analysis (SABiosciences), FCL treatment produced a 4.51-fold reduction in VEGF expression in the 0.25 mg/ml FCL-treated tumors compared to the untreated tumors in the CAM model (p = 0.002, 95 % CI = 0.08, 0.37) (Fig. 6).
Fig. 6.
Alteration in VEGF expression level in GBM tumors before and after FCL treatment in the CAM model.
FCL Enhances the Effect of TMZ
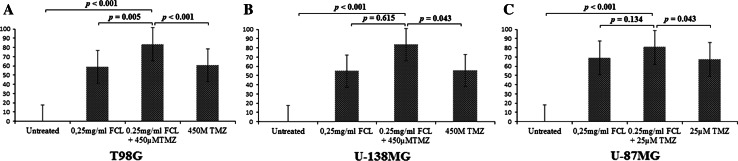
To evaluate the utility of FCL as a supplement to chemotherapy, we analyzed potential interactions between FCL and the most commonly used chemotherapeutic agent, TMZ. The T98G and U-138 MG cells were treated with 450 µM of TMZ, and the U-87 MG cells were treated with 25 µM of TMZ in the absence or presence of 0.25 mg/ml FCL. The WST-1 assay was performed after 24 h of incubation, and drug interactions were evaluated based on the reduction in proliferation (Fig. 6). The IC50 of FCL for all cell lines cells was 0.25 mg/ml. When the T98G and U-138 MG cells were treated with 450 µTMZ, the reduction in proliferation was determined to be 60.5 and 55.5 %, respectively, and when the U-87 MG cells were treated with 25 µTMZ, the reduction in proliferation was determined to be 67.1 % compared to the untreated culture. When the T98G, U-138 MG, and U-87 MG cells were treated with TMZ in the presence of 0.25 mg/ml FCL, the reduction in proliferation was determined to be 83.1, 83.4, and 80.6 %, respectively, compared to the untreated culture. The results presented in Fig. 7 show that the addition of FCL affected the toxicity of the applied TMZ. The responsiveness to TMZ was intensified in the presence of the extract. This result indicated that FCL has synergistic effects with the toxicity of TMZ.
Fig. 7.
Effect of FCL and TMZ concentration on cell viability. (P values evaluated with One Way ANOVA and Tukey’s analyses using SPSS 16.00 software for Windows (IBM, Chicago, IL)).
miRNA expression profiles in T98G cells treated with FCL or TMZ
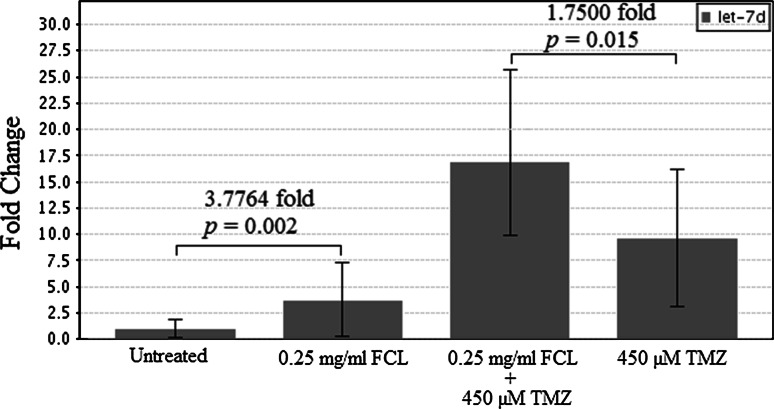
To evaluate the modulating effect of FCL on the expression pattern of GBM progression-related miRNAs, 40 miRNAs were screened in T98G cells after treatment with 0.25 mg/ml FCL, 450 µM of TMZ, or a combination of 450 µM TMZ and 0.25 mg/ml FCL for 24 h. According to the RT2 Profiler PCR Array Data Analysis (SABiosciences), TMZ treatment differentially upregulated the expression of let-7d, and FCL synergistically enhanced this TMZ effect (Table 2). Depend on RT-PCR analysis of T98G cells after treatment with 0.25 mg/ml FCL, 450 µM of TMZ, or a combination of 450 µM TMZ and 0.25 mg/ml FCL, let-7d expression was 3.77 fold increased in 0.25 mg/ml FCL in compare to untreated cells. In addition, a combination of 450 µM TMZ and 0.25 mg/ml FCL caused 1.75 fold more expression of let-7d than cells treated with 450 µM of TMZ (Fig. 8). These results indicate that the compounds present in the FCL specifically altered the expression of let-7d in comparison with TMZ alone (Fig. 8).
Fig. 8.
Alteration in let-7d expression level in T98G cells after FCL or TMZ treatment depends on ten repeat of RT-PCR analyses (p values evaluated with the Independent sample t test using RT2 Profiler PCR Array Data Analysis)
Discussion
Despite recent advances in treatment, long-term survival of GBM remains poor. Current therapy is nonspecific, and the success of preventing recurrence varies between cases. Therefore, there is an increasing need for alternative and adjuvant treatment options. In this context, there is great interest in dietary plants with their chemopreventive and chemotherapeutic potential (Khor et al. 2006). Ficus carica has been traditionally used for its medicinal benefits for metabolic, cardiovascular, respiratory, spasmodic, and inflammatory diseases (Mawa et al. 2013). The inhibitory effect of FCL on the proliferation of various cancer cell lines such as T-cell leukemia, Burkitt B cell lymphoma, melanoma, prostate cancer, and mammary and stomach cancers has been demonstrated (Rubnov et al. 2001; Hashemi et al. 2011; Menichini et al. 2012). However, the effect of this extract on brain tumors is unknown. In the current study, we evaluated the anti-carcinogenic activity of FCL on GBM cell lines. To analyze the potential anti-cancer effect of FCL in GBM tumors with different characteristics that affect tumor aggressiveness, such as the TMZ response, we first evaluated the cytotoxic effect of FCL on T98G, U-138 MG, and U-87 MG cell lines using WST-1 analyses. T98G is a TMZ-resistant GBM cell line and is heterozygous for MGMT methylation. U-138 MG cells are highly resistant to TMZ with an unmethylated MGMT gene. Moreover, U-87 MG cells are TMZ-sensitive, and the MGMT gene is methylated (Yoshino et al. 2010). According to WST-1 analyses, the optimal activity of FCL was observed in 24 h. Therefore, 24 h FCL treatments were performed in the subsequent analyses. In addition, the IC50 of FCL for all cells was 0.25 mg/ml. At a concentration of 0.25 mg/ml, FCL caused a significant decrease in the proliferation of T98G (58.7 %), U-138 MG cells (54.94 %), and U-87 MG cells (68.81 %) at 24 h (p < 0.05). Although the T98G, U-138 MG, and U-87 MG cell lines differ with respect to their MGMT methylation status, the IC50s of FCL in these cell lines were similar. Therefore, we suggest that FCL may cause cell death via an MGMT-independent pathway. Moreover, 0.25 mg/ml FCL caused a 14.83 ± 0.33 % inhibition of lymphocytes. This finding demonstrates the low side effects of FCL on non-tumor cells.
The molecular mechanism of the action of FCL on tumor cell viability was evaluated using Annexin-V-FITC/PI and TUNEL assays. These analyses displayed the apoptotic activity of FCL. Based on HPLC analyses, the FCL that was used in this study contained 28.784 ± 0.080 mg/L protocatechuic acid. The apoptotic activity of protocatechuic acid derived from Matricaria chamomilla and Uncaria tomentosa was demonstrated in HL-60 leukemia cells in a previous study (Anter et al. 2011). Although the current findings are unique regarding the molecular activity of FCL because the major phenolic content of our extract is protocatechuic acid, our data support previous findings. Thus, this study suggests that that apoptosis inductive property of FCL on GBM cell lines could be partially responsible for its anti-cancer activity.
Secondly, according to CAM assay findings, FCL treatment caused a reduction in the weight of the tumors (p = 0.08) and a reduction in the VEGF expression level (p = 0.002). Moreover, the effect of FCL on invasion was analyzed in only GBM tumors derived from the T98G cells in the current CAM model. Because T98G cells do not respond well to TMZ, these data are also evidence of the anti-cancer effect of FCL in drug resistant GBM tumors. Based on previous studies, plants extracts that contain protocatechuic acid may potentially inhibit invasion (Yin et al. 2009; Lin et al. 2011; Spilioti et al. 2014). Spilioti et al. revealed the anti-cancer activity of protocatechuic acid derived from honey on prostate cancer (PC-3) and breast cancer (MCF-7) cells. Based on that study, there was a negative correlation between the protocatechuic acid content of honey and vascular cell adhesion molecule 1 (VCAM-1) expression (Spilioti et al. 2014), which also promotes macrophage cell interaction and tumor cell invasion in GBM (Zheng et al. 2013). These data may imply the potential effect of FCL on GBM invasion via its protocatechuic acid content. However, a recent study reported the opposite findings; according to Khang et al., protocatechuic acid promotes angiogenesis via programmed PI3 K/Akt/eNOS/VEGF signaling (Kang et al. 2013). Conversely, our findings regarding FCL, which is a plant that contains protocatechuic acid, defined the inhibitory effect of FCL on angiogenesis in GBM. Khang et al. analyzed the activity of protocatechuic acid in a human brain microvascular endothelial cell line that is a type of non-tumor cell. Similarly, we displayed a very low-level cytotoxic effect of FCL on lymphocytes. Although further studies are required to clarify this mechanism, these data imply the function of protocatechuic acid in the invasion in a cell type-dependent manner.
Because the most common chemotherapeutic agent used in GBM patients is TMZ, we evaluated the effect of FCL as a supplement to TMZ on GBM cell lines. The T98G and U-138 MG cells were treated with 450 µM, and the U-87 MG cells were treated with 25 µM of TMZ in the absence or presence of 0.25 mg/ml FCL. According to our WST-1 analysis findings, the viability rate of all tumor cells was significantly lower after treatment with FCL-TMZ combination than the rate after treatment with TMZ alone (p > 0.01, = 0.043, and = 0.043 in T98G, U-138 MG, and U-87 MG, respectively). These results revealed that the addition of FCL is synergistic with the therapeutic activity of TMZ. To clarify the molecular mechanism of this effect, 40 selected miRNAs related to GBM development and drug resistance were screened using T98G cells treated with FCL, TMZ, or a combination of TMZ and FCL. This analysis showed that the expression of let-7d was significantly altered after FCL treatment. This finding was also validated with RT-PCR analysis of let-7d after FCL treatment. This analyses showed that let-7d expression was 3.77 fold upregulated compared to untreated cells (p = 0.002). Furthermore, TMZ treatment in combination with FCL produced a 1.75-fold increased expression of let-7d compared to the same dose of TMZ alone (p = 0.015). Evidences have indicated that nutrition as a lifestyle factor may provide health benefits and cancer prevention through epigenetic-modifying properties with their phenolic compounds (Kong et al. 2013; Sanli et al. 2013). In a previous study of Paluszczak et al., the inhibitor effect of protocatechuic acid on DNMT activity was defined (Paluszczak et al. 2010). In addition, Wang et al. demonstrated that protocatechuic acid at physiological concentrations represses macrophage miR-10b (Wang et al. 2012). According to HPLC analysis, the major phenolic compound of water fraction of FCL is protocatechuic acid. Thus, our findings imply that the protocatechuic acid content of FCL may cause an induction in let-7d expression. Although previous studies strongly indicated the effect of protocatechuic acid on epigenetic mechanisms, further biochemical analyses will clarify the mechanism of FCL and let-7d interaction in more detail.
The Let-7 family has broad tumor suppressor function, and the members of this family are downregulated in many cancer types compared to normal tissue and during tumor progression (Takamizawa et al. 2004, Dahiya et al. 2008; O’Hara et al. 2009). One of the well-studied target genes of let-7d is HMGA2 (Xi et al. 2014). Xi et al. demonstrated a reduction in Hmga2 level in the presence of let-7 family members (Xi et al. 2014). Hmga2 is a transcription factor which plays a role in regulating of epithelial mesenchymal transition (EMT) (Thuault et al. 2006). Transforming growth factor beta (TGF-b) induces EMT by regulating the expression and activity of several transcription factors, such as Snail/Slug, ZEB1/2, and Twist proteins, the expression of which are all interconnected (Heldin et al. 2012; Ishikawa et al. 2014). These proteins repress E-cadherin expression and induce mesenchymal genes, being central to the EMT processes (Peinado et al. 2007; Ishikawa et al. 2013). HMGA2 is critically involved in these nuclear events during EMT induced by TGF-b. HMGA2 is trans activated by Smads and in turn upregulates Snail and Twist in cooperation with Smads, leading to upregulation of other members of the regulators and the consequent nuclear reprogramming (Heldin et al. 2012; Ishikawa et al. 2013). The relationship between high HMGA2 levels and invasion was demonstrated in ovarian cancer and retinoblastomas in previous studies (Xi et al. 2014; Mu et al. 2010). Recent studies also showed that over expression of HMGA2 may participate in regulating tumor cell invasion in high grade gliomas (Chen et al. 2013). Another well-known target of let-7d is Signal Transducer and Activator of Transcription 3 (STAT3) which is known to promote tumor cell proliferation, survival, and invasion (Ranger et al. 2009; Barbieri et al. 2010). Stat3 proteins suppress by let-7 family (Sugimura et al. 2012) and regulate the common downstream targets, such as cyclin D1, Bcl2 family proteins, Rho family GTPases, hypoxia-inducible factor-1α, and VEGF (Zushi et al. 1998; Teng et al. 2009; Xu et al. 2005; Fang 2014). In the current study, we detected the inductive effect of the FCL–TMZ combination on let-7d expression. Although we did not analyze the effect of FCL on the expression level of HMGA2 as a target gene of let-7d, in support of our data, we determined the inhibitory effect of FCL on tumor weight and VEGF expression level in the CAM assay. In a previous study, Chen et al. defined a significant correlation between HMGA2 and VEGFA expression levels in gliomas (Chen et al. 2013). Therefore, our data imply that FCL may reduce invasion via induction of let-7d expression and decreasing HMGA2 and VEGF expression, and the responsiveness to TMZ may be intensified in the presence of FCL (Fig. 9).
Fig. 9.
a schematic summarizing the hypothesis of the effect of the FCL–TMZ interaction on let-7d expression during invasion. A. Induction of invasion with HMGA2 and VEGFA expression. b. Inhibition of HMGA2 and VEGFA via induction of let-7d expression by FCL–TMZ treatment relieves invasion
In summary, in the present study, treatment with 0.25 mg/ml FCL caused cytotoxicity in the T98G, U-138 MG, and U-87 MG cells. Although FCL causes cell death via both apoptosis and necrosis, the cytotoxic activity of this extract is negligible in non-tumor cells. In addition, when we evaluate the effect of FCL on the regulation of miRNAs, we found that the use of FCL in combination with TMZ increased the rate of inhibition of cell proliferation in GBM and increased the expression levels of let-7d, which is involved in the regulation of invasion compared to cells treated with only TMZ. Therefore, taking all the data together, we believe that FCL may synergistically affect the activity of TMZ in GBM tumors and reduce invasion with less cytotoxic activity in non-tumor cells.
In conclusion, to the best of our knowledge, our data are the first to demonstrate that FCL causes cell death in GBM cells with different responses to TMZ and that this effect is synergistically increased in combination with TMZ. In addition, the current study is the first to demonstrate the effect of FCL on modulation of let-7d expression, which may be an important mechanism underlying the anti-invasive effect of this extract. Even purification and identification of the active compounds of FCL are required for a better understanding of the involved protective mechanisms and for possible future clinical application; although further in vivo experiments, miRNA transfection and protein analysis are necessary to validate our data, we suggest that FCL may be a strong candidate for studies of therapeutic cancer drugs.
Acknowledgments
This work was supported by the Science Foundation of Uludag University (Project number; UAP(T)-2012/2 and HDP(T)-2013/3). This study was performed as part of G. Tezcan’s PhD thesis project.
Conflict of interest
None.
References
- Ahmed W, Khan AQ, Malik A (1988) Two triterpenes from the leaves of Ficus carica. Planta Med 54(5):481 [DOI] [PubMed] [Google Scholar]
- Anter J, Romero-Jiménez M, Fernández-Bedmar Z, Villatoro-Pulido M, Analla M, Alonso-Moraga A, Muñoz-Serrano A (2011) Antigenotoxicity, cytotoxicity, and apoptosis induction by apigenin, bisabolol, and protocatechuic acid. J Med Food 14(3):276–283 [DOI] [PubMed] [Google Scholar]
- Barbieri I, Pensa S, Pannellini T, Quaglino E, Maritano D, Demaria M, Voster A, Turkson J, Cavallo F, Watson CJ, Provero P, Musiani P, Poli V (2010) Constitutively active Stat3 enhances neu-mediated migration and metastasis in mammary tumors via upregulation of Cten. Cancer Res 70(6):2558–2567 [DOI] [PubMed] [Google Scholar]
- Canal JR, Torres MD, Romero A, Pérez C (2000) A chloroform extract obtained from a decoction of Ficus carica leaves improves the cholesterolaemic status of rats with streptozotocin-induced diabetes. Acta Physiol Hung 87(1):71–76 [DOI] [PubMed] [Google Scholar]
- Chen Z, Cheng Q, Ma Z, Xi H, Peng R, Jiang B (2013) Overexpression of RKIP inhibits cell invasion in glioma cell lines through upregulation of miR-98. Biomed Res Int. 2013:695179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conforti F, Menichini G, Zanfini L, Tundis R, Statti GA, Provenzano E, Menichini F, Somma F, Alfano C (2012) Evaluation of phototoxic potential of aerial components of the fig tree against human melanoma. Cell Prolif. 45:279–285. doi:10.1111/j.1365-2184.2012.00816.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahiya N, Sherman-Baust CA, Wang TL, Davidson B, Shih IeM, Zhang Y, Wood W 3rd, Becker KG, Morin PJ (2008) MicroRNA expression and identification of putative miRNA targets in ovarian cancer. PLoS One 3(6):e2436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Leva G, Croce CM (2010) Roles of small RNAs in tumor formation. Trends Mol Med 16:257–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang B (2014) Genetic interactions of STAT3 and anticancer drug development. Cancers (Basel) 6(1):494–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandellini P, Profumo V, Folini M, Zaffaroni N (2011) MicroRNAs as new therapeutic targets and tools in cancer. Expert OpinTher Targets 15:265–279 [DOI] [PubMed] [Google Scholar]
- Hashemi SA, Abediankenari S, Ghasemi M, Azadbakht M, Yousefzadeh Y, Dehpour AA (2011) The effect of fig tree latex (Ficus carica) on stomach cancer line. Iran Red Crescent Med J. 13(4):272–275 [PMC free article] [PubMed] [Google Scholar]
- Heldin CH, Vanlandewijck M, Moustakas A (2012) Regulation of EMT by TGFβ in cancer. FEBS Lett 586(14):1959–1970 [DOI] [PubMed] [Google Scholar]
- Hulleman E, Helin K (2005) Molecular mechanisms in gliomagenesis. Adv Cancer Res 94:1–27 [DOI] [PubMed] [Google Scholar]
- Ishikawa F, Kaneko E, Sugimoto T, Ishijima T, Wakamatsu M, Yuasa A, Sampei R, Mori K, Nose K, Shibanuma M (2014) A mitochondrial thioredoxin-sensitive mechanism regulates TGF-β-mediated gene expression associated with epithelial-mesenchymal transition. Biochem Biophys Res Commun 443(3):821–827 [DOI] [PubMed] [Google Scholar]
- Kang Z, Zhu H, Jiang W, Zhang S (2013) Protocatechuic acid induces angiogenesis through PI3K-Akt-eNOS-VEGF signalling pathway. Basic Clin Pharmacol Toxicol 113(4):221–227 [DOI] [PubMed] [Google Scholar]
- Khor TO, Keum YS, Lin W, Kim JH, Hu R, Shen G, Xu C, Gopalakrishnan A, Reddy B, Zheng X, Conney AH, Kong AN (2006) Combined inhibitory effects of curcumin and phenethyl isothiocyanate on the growth of human PC-3 prostate xenografts in immunodeficient mice. Cancer Res 66(2):613–621 [DOI] [PubMed] [Google Scholar]
- Kong AN, Zhang C, Su ZY (2013) Targeting epigenetics for cancer prevention by dietary cancer preventive compounds—the case of miRNA. Cancer Prev Res (Phila). 6(7):622–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krex D, Klink B, Hartmann C, von Deimling A, Pietsch T, Simon M, Sabel M, Steinbach JP, Heese O, Reifenberger G, Weller M, Schackert G (2007) Long-term survival with glioblastoma multiforme. Brain 130:2596–2606 [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta DeltaC(T)) Method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114:97–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawa S, Husain K, Jantan I (2013) Ficus carica L. (Moraceae): phytochemistry, traditional uses and biological activities. Evid Based Complement Alternat Med 2013:974256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menichini G, Alfano C, Provenzano E, Marrelli M, Statti GA, Somma F, Menichini F, Conforti F (2012) Fig latex (Ficus carica L. cultivar Dottato) in combination with UV irradiation decreases the viability of A375 melanoma cells in vitro. Anticancer Agents Med Chem. 12(8):959–965 [DOI] [PubMed] [Google Scholar]
- Mijatovic SA, Timotijevic GS, Miljkovic DM, Radovic JM, Maksimovic- Ivanic DD, Dekanski DP, Stosic-Grujicic SD (2011) Multiple antimelanoma potential of dry olive leaf extract. Int J Cancer 128:1955–1965 [DOI] [PubMed] [Google Scholar]
- Mu G, Liu H, Zhou F, Xu X, Jiang H, Wang Y, Qu Y (2010) Correlation of overexpression of HMGA1 and HMGA2 with poor tumor differentiation, invasion, and proliferation associated with let-7 down-regulation in retinoblastomas. Hum Pathol 41(4):493–502 [DOI] [PubMed] [Google Scholar]
- Newman DJ, Cragg GM (2007) Natural products as sources of new drugs over the last 25 years. J Nat Prod 70:461–477 [DOI] [PubMed] [Google Scholar]
- O’Hara AJ, Wang L, Dezube BJ, Harrington WJ Jr, Damania B, Dittmer DP (2009) Tumor suppressor microRNAs are underrepresented in primary effusion lymphoma and Kaposi sarcoma. Blood 113:5938–5941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paluszczak J, Krajka-Kuzniak V, Baer-Dubowska W (2010) The effect of dietary polyphenols on the epigenetic regulation of gene expression in MCF7 breast cancer cells. Toxicol Lett 192(2):119–125 [DOI] [PubMed] [Google Scholar]
- Peinado H, Olmeda D, Cano A (2007) Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer 7(6):415–428 [DOI] [PubMed] [Google Scholar]
- Ranger JJ, Levy DE, Shahalizadeh S, Hallett M, Muller WJ (2009) Identification of a Stat3-dependent transcription regulatory network involved in metastatic progression. Cancer Res 69(17):6823–6830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubnov S, Kashman Y, Rabinowitz R, Schlesinger M, Mechoulam R (2001) Suppressors of cancer cell proliferation from fig (Ficus carica) resin: isolation and structure elucidation. J Nat Prod 64(7):993–996 [DOI] [PubMed] [Google Scholar]
- Sanli T, Strano S, Muti P (2013) Lifestyle factors and microRNAs: a new paradigm in cancer chemoprevention. Microrna 2(2):82–90 [DOI] [PubMed] [Google Scholar]
- Serraclara A, Hawkins F, Pérez C, Domínguez E, Campillo JE, Torres MD (1998) Hypoglycemic action of an oral fig-leaf decoction in type-I diabetic patients. Diabetes Res Clin Pract 39(1):19–22 [DOI] [PubMed] [Google Scholar]
- Sethi S, Li Y, Sarkar FH (2013) Regulating miRNA by natural agents as a new strategy for cancer treatment. Curr Drug Targets 14(10):1167–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spilioti E, Jaakkola M, Tolonen T, Lipponen M, Virtanen V, Chinou I, Kassi E, Karabournioti S, Moutsatsou P (2014) Phenolic acid composition, antiatherogenic and anticancer potential of honeys derived from various regions in Greece. PLoS One 9(4):e94860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO, European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups, National Cancer Institute of Canada Clinical Trials Group (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996 [DOI] [PubMed] [Google Scholar]
- Sugimura K, Miyata H, Tanaka K, Hamano R, Takahashi T, Kurokawa Y, Yamasaki M, Nakajima K, Takiguchi S, Mori M, Doki Y (2012) Let-7 expression is a significant determinant of response to chemotherapy through the regulation of IL-6/STAT3 pathway in esophageal squamous cell carcinoma. Clin Cancer Res 18(18):5144–5153 [DOI] [PubMed] [Google Scholar]
- Takamizawa J, Konishi H, Yanagisawa K, Tomida S, Osada H, Endoh H, Harano T, Yatabe Y, Nagino M, Nimura Y, Mitsudomi T, Takahashi T (2004) Reduced expression of the let-7 microRNAs in human lung cancers in association with shortened postoperative survival. Cancer Res. 1 64(11):3753–3756 [DOI] [PubMed] [Google Scholar]
- Teng TS, Lin B, Manser E, Ng DC, Cao X (2009) Stat3 promotes directional cell migration by regulating Rac1 activity via its activator betaPIX. J Cell Sci 122(Pt 22):4150–4159 [DOI] [PubMed] [Google Scholar]
- Thuault S, Valcourt U, Petersen M, Manfioletti G, Heldin CH, Moustakas A (2006) Transforming growth factor-beta employs HMGA2 to elicit epithelial mesenchymal transition. J Cell Biol 174:175–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunca B, Tezcan G, Cecener G, Egeli U, Ak S, Malyer H, Tumen G, Bilir A (2012) Olea europaea leaf extract alters microRNA expression in human glioblastoma cells. J Cancer Res Clin Oncol 138(11):1831–1844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Xia M, Yan X, Li D, Wang L, Xu Y, Jin T, Ling W (2012) Gut microbiota metabolism of anthocyanin promotes reverse cholesterol transport in mice via repressing miRNA-10b. Circ Res 111(8):967–981 [DOI] [PubMed] [Google Scholar]
- Xi YN, Xin XY, Ye HM (2014) Effects of HMGA2 on malignant degree, invasion, metastasis, proliferation and cellular morphology of ovarian cancer cells. Asian Pac J Trop Med 7(4):289–292 [DOI] [PubMed] [Google Scholar]
- Xu Q, Briggs J, Park S, Niu G, Kortylewski M, Zhang S, Gritsko T, Turkson J, Kay H, Semenza GL, Cheng JQ, Jove R, Yu H (2005) Targeting Stat3 blocks both HIF-1 and VEGF expression induced by multiple oncogenic growth signaling pathways. Oncogene 24(36):5552–5560 [DOI] [PubMed] [Google Scholar]
- Lin HH, Chen JH, Chou FP, Wang CJ (2011) Protocatechuic acid inhibits cancer cell metastasis involving the down-regulation of Ras/Akt/NF-κB pathway and MMP-2 production by targeting RhoB activation. Br J Pharmacol. 162(1):237–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez C, Canal JR, Campillo JE, Romero A, Torres MD (1999) Hypotriglyceridaemic activity of Ficus carica leaves in experimental hypertriglyceridaemic rats. Phytother Res. 13(3):188–191 [DOI] [PubMed] [Google Scholar]
- Yin MC, Lin CC, Wu HC, Tsao SM, Hsu CK (2009) Apoptotic effects of protocatechuic acid in human breast, lung, liver, cervix, and prostate cancer cells: potential mechanisms of action. J Agric Food Chem 57(14):6468–6473 [DOI] [PubMed] [Google Scholar]
- Yoshino A, Ogino A, Yachi K, Ohta T, Fukushima T, Watanabe T, Katayama Y, Okamoto Y, Naruse N, Sano E, Tsumoto K (2010) Gene expression profiling predicts response to temozolomide in malignant gliomas. Int J Oncol 36:1367–1377 [DOI] [PubMed] [Google Scholar]
- Zheng Y, Yang W, Aldape K, He J, Lu Z (2013) Epidermal growth factor (EGF)-enhanced vascular cell adhesion molecule-1 (VCAM-1) expression promotes macrophage and glioblastoma cell interaction and tumor cell invasion. J Biol Chem 288(44):31488–31495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zushi S, Shinomura Y, Kiyohara T, Miyazaki Y, Kondo S, Sugimachi M, Higashimoto Y, Kanayama S, Matsuzawa Y (1998) STAT3 mediates the survival signal in oncogenic ras-transfected intestinal epithelial cells. Int J Cancer 78(3):326–330 [DOI] [PubMed] [Google Scholar]