Table 1.
Impact of size and hydrophobicity of carboxamide derivatives derived from the DECL hit (A127/B178) on Sirt6 deacylase activity.
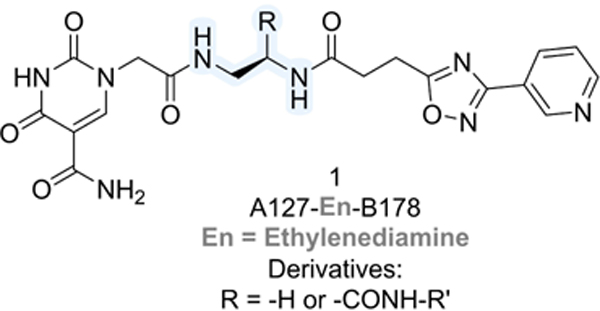
| ||
|---|---|---|
| Cmpd | Substituent R = | Inhibition c = 10 μM |
| 1 | H | 0% |
| 2-Me |

|
40%[a] |
| 2-Pr |
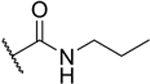
|
60% (IC50 = 8.9 μM)[b] pIC50 (5.1 ± 0.03) |
| 2-MeOEt |
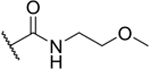
|
9% |
| 2-PhEt |
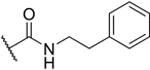
|
29% |
| 2-Bn |

|
30%[a] |
| 2-Pip |

|
28% |
| 2-Ph |
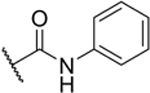
|
53% (IC50 = 9.3 μM) pIC50 (5.0 ± 0.05) |
| 2-CyBu |
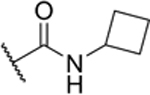
|
64% (IC50 = 8.2 μM) pIC50 (5.1 ± 0.05) |
| 3 |

|
3% |
| Nicotinamide (1000 μM) | 92% | |
Sirt6 activity measured at c = 40 μM.
Previously measured IC50 = 6.7 μM Ref.[20] The biochemical assay used to assess the in vitro activity of the compounds was done in duplicate and repeated twice on different days.
