Abstract
The intestinal tract, which is the primary site of digestion and absorption of nutrients, is one of the most vulnerable organs during aging. Dietary nitrate, which is mainly derived from the diet and absorbed in the intestinal tract, is a key messenger that connecting oral and general health. However, whether dietary nitrate regulates intestinal tract homeostasis remains unclear. Our data revealed that the serum and salivary nitrate levels decreased during mice aging. The functional proteins of the epithelial barrier (E-cadherin, Claudin-1 and Zonula Occludens-1) in the colon tissues decreased during the aging process. Long-term nitrate supplement in drinking water restored the serum and salivary nitrate levels and increased the functional proteins expression of the colon epithelial barrier. Dietary nitrates increase the relative abundance of some intestinal probiotics, particularly those associated with the production of short-chain fatty acids, such as Blautia, Alloprevotella, Butyricicoccus, and Ruminococcaceae, while promoting the butyric acid production in the colon. Moreover, the expression of Sialin (encoded by Slc17a5), which is a nitrate transporter, increased in the colon epithelial cells by nitrate supplementation. The epithelial cell-conditional Slc17a5-knockout mutant mice (K14-cre; Slc17a5fl/fl) revealed that the functional proteins expression of the colon epithelial barrier and the proliferation of PCNA-positive intestinal epithelial cells in the colon crypts was significantly decreased compared with those of the K14-cre; Slc17a5fl/+ mice. Taken together, our findings suggested that nitrate supplementations were associated with the increased expression of colonic epithelial barriers-related proteins and the increased Sialin expression. Nitrate may serve as a potential therapeutic approach in maintaining aged colonic homeostasis.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10522-024-10127-5.
Keywords: Aging, Nitrate, Sialin, Colon homeostasis, Microbiota, Metabolite
Introduction
Aging, which is a multifactorial process characterized by the progressive decline of gastrointestinal function, which adversely affects nutrient absorption and excretion, leading to the development of systemic diseases (Funk et al. 2020; Haran and McCormick 2021; Keebaugh and Ja 2017; An et al. 2018). A healthy intestine maintains functional homeostasis, which is a dynamic equilibrium state maintained by the intestinal epithelial barrier, immune system, microbiota, and metabolites (Keebaugh and Ja 2017; An et al. 2018). Age-related intestinal barrier dysfunction is characterized by the inability to maintain selective permeability (Parrish 2017). The composition of the intestinal microbiota greatly changes with age, with a decrease and increase in the abundance of beneficial and pathogenic microorganisms, respectively (Funk et al. 2020; O’Toole and Jeffery 2015; Buford 2017). These changes further damage the intestinal epithelial permeability, thereby affecting body health (O’Toole and Jeffery 2018; Zapata and Quagliarello 2015). Since the gradual loss of health caused by aging, we should shift our focus to maintaining, restoring and improving our health (Rattan 2024). Therefore, maintaining intestinal homeostasis is being considered as an intervention for alleviating intestine aging.
Increasing evidence has suggested that dietary interventions, including intermittent fasting, balanced protein and fiber intake, and vegetarian diets, can palliate age-related diseases (Brandhorst et al. 2015; Duregon et al. 2023; Gill et al. 2021; Sbierski-Kind et al. 2022). Nitrate, which is an inorganic anion abundantly found in green leafy vegetables, acts as a messenger linking oral and systemic health. Most of the dietary nitrate absorbed into the blood through the intestinal mucosa is excreted in the urine; further, approximately 25% of the dietary nitrate intake is absorbed and secreted through the salivary glands for maintaining the body nitrate levels (Jones et al. 2018; Lundberg et al. 2018; Xia et al. 2003). Nitrate is subsequently reduced to nitric oxide (NO) by commensal facultative anaerobic bacteria and various reductases in the mouth and tissues (Lundberg et al. 2008). Sialin (encoded by Slc17a5), which is a nitrate transporter on the mammalian cell membrane, mediates nitrate transport into the cells and is critically involved in maintaining NO homeostasis (Wang and Qin 2022; Qu et al. 2016; Qin et al. 2012). Sialin is highly expressed in various organs in the body, including the salivary glands, brain, and colon. The salivary gland is crucially involved in regulating the nitrate levels, with nitrate reciprocally regulating salivary gland function (Wang and Qin 2022). Specifically, nitrate promotes Sialin expression in the salivary glands and improves irradiation-induced salivary gland injury (Feng et al. 2021). A recent study reported that reduced Sialin expression in rats with type 2 diabetes, which was accompanied by reduced nitrate and nitrite levels (Yousefzadeh et al. 2023). Nitrate administration alleviated type 2 diabetes by increasing the NO levels (Khorasani et al. 2019). The interaction between nitrate and Sialin facilitates the maintenance of the NO levels, and consequently body homeostasis.
We previously reported that dietary nitrate significantly reduced gastric mucosal damage under stress via the nitrate-nitrite-NO pathway (Jin et al. 2013). Moreover, nitrate ameliorates colitis induced by dextran sodium sulfate by regulating intestinal microbiota homeostasis (Hu et al. 2020). Other studies have found that nitrate can prevent aging-related liver damage by reducing the serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels (Wang et al. 2018). In addition, nitrate efficiently prevents total body irradiation-induced colon epithelium injury by suppressing the oxidative stress (Wang et al. 2020a, b). The recent study showed that nitrate can prevent hypoxia-induced intestinal jury through activating the nuclear translocation of Hif1α (Xu et al. 2024). These findings suggest that nitrate may serves as a novel approach to protect gastrointestinal function. However, whether dietary nitrate maintains intestinal homeostasis during aging remains unclear.
This study aimed to investigate the function of long-term nitrate supplementation on age-related intestinal dyshomeostasis in a naturally aging mouse model, as well as the mechanism of intestinal homeostasis regulation by nitrate in conditional Slc17a5 knockout mice. Our results showed that the reduction of nitrate levels correlated with age-related intestine epithelial dysfunction. Long-term nitrate supplementation restores intestinal function homeostasis by promoting Sialin expression, thereby delaying aged-related intestinal dyshomeostasis.
Materials and methods
Animals
The experimental part was approved by the Animal Care and Use Committee of Capital Medical University (Ethical Code: No. AEEI-2020-197). All animals were maintained under specific-pathogen-free conditions and had free access to water and food. All experiments were conducted in accordance with the National Institute of Health guidelines for Animal care and use. All animals were sacrificed with an anaesthetic overdose at certain times. All animals had C57BL/6 background.
6 weeks old C57BL/6 were purchased from Beijing SPF Biotechnology co., Ltd. Slc17a5-floxed allele (Slc17a5fl/fl) mice and K14-Cre-ERT mice were purchased from the Cyagen Bioscience Inc. Cre-ERT is a Cre-recombinant enzyme that fuses with the estrogen receptor, which binds to tamoxifen and is transferred to the nucleus. Keratin14 (K14) is a marker for epithelial cells, and in K14-Cre-ERT mice, Cre-ERT expression is targeted to K14-expressing epithelial tissue. The K14-Cre mice were crossed with the Slc17a5fl/fl mice to generate K14-Cre; Slc17a5fl/fl mice, and epithelial cell-specific knockout of K14-Cre; Slc17a5fl/fl was induced by intraperitoneal injection of tamoxifen with a single injection at 200 mg/kg body weight. The K14-Cre; Slc17a5fl/+ C57BL/6 (8-week-old) mice were used as controls. Mouse tails tips were obtained at 10 days after birth, and DNA were extracted for PCR genotype identification.
The 6-week-old mice were fed normally to 18 months and randomly divided into two groups: Aged (normal water) and Aged + nitrate group (4 mM nitrate was added to drinking water), with 10 mice (half male and half female) in each group. Sodium nitrate (NaNO3; S8170; Sigma-Aldrich) was dissolved with drinking water as previously reported (Xu et al. 2024). Aged and aged + nitrate group were observed for 6 months. The 8-weeks-old mice were used as the young control group and were given normal drinking water. At the end of the experiment, saliva, blood, colon tissue and feces were collected for follow-up experiments.
Detection of nitrate levels
Saliva and blood samples were collected at the end of the experiment. Saliva samples were filtered using ultrafiltration tubes, and serum was obtained by centrifugation of the blood samples at 3000×g for 20 min at room temperature. Concentrations of nitrate were checked using a Total Nitric Oxide and Nitrate/Nitrite Parameter Assay Kit following the manufacturer’s protocol.
Histological analyses
Tissues were fixed with 4% paraformaldehyde, dehydrated with gradient ethanol and then embedded in paraffin. Sections (4 μm thickness) were stained with haematoxylin and eosin (H&E) staining and examined using microscope (OLYMPUS).
Immunofluorescence (IF) staining
The sections were deparaffinized with xylene and rehydrated with a series of ethanol solutions in phosphate-buffered saline (PBS). After that, the tissue sections were processed with antigen retrieval by boiling the slides in sodium citrate buffer (10 mmol/L, pH 6.0) for 20 min. Then, the sections were immersed in 10% H2O2/methanol for 10 min to block the endogenous peroxidases activity, followed by PBS with 5% BSA and 0.2% Triton X-100 at room temperature for 1 h. The sections were incubated with primary antibodies overnight at 4 °C. After washing with PBS for 10 min three times, sections were incubated with secondary antibodies (Alexa Fluor or horseradish peroxidase-conjugated series) at room temperature for 2 h. IF images were taken using a Leica confocal microscope.
The primary antibodies used were as follows: rabbit polyclonal anti-claudin 1 (1:100 dilution, 71-7800, Invitrogen), mouse monoclonal anti-ZO-1 (1:200 dilution, 33-9100, Thermo Fisher Scientific), mouse monoclonal anti-E cadherin (1:200 dilution, ab231303, Abcam), mouse monoclonal anti-Lgr5 (1:100 dilution, MA5-25644, Thermo Fisher Scientific), rabbit monoclonal anti-PCNA (1:1000 dilution, ab92552, Abcam), rabbit polyclonal anti-SLC17A5 (1:100 dilution, PA5-30517, Thermo Fisher Scientific) and rabbit polyclonal anti-MUC2 (1:500 dilution, 27675-1-AP, Proteintech).
The secondary antibodies used were as follows: donkey polyclonal anti-rabbit IgG (H + L) Alexa Fluor 594 (1:1000 dilution, A-21207; Thermo Fisher Scientific), donkey polyclonal anti-mouse IgG (H + L) and Alexa Fluor 488 (1:1000 dilution, A-21202; Thermo Fisher Scientific).
The negative controls were as follows: PBS instead of the primary antibody was used as the secondary antibody only control. Mouse monoclonal anti-IgG (1:200 dilution, SC-51643, Santa Cruz Biotechnology) instead of the primary antibody was used as negative control.
Western blot assay
The 40 mg colon tissues were completely cleanout under PBS and without scraped crypts, homogenized in RIPA lysis and Extraction Buffer (Thermo Fisher Scientific). The Colon tissue samples were randomly taken from 3 animals in each group. Individual colon lysates were loaded and separated by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to an Immobilon-P Polyvinylidene difluoride membrane (Millipore). After blocking with 5% milk in PBS, the membrane was incubated with primary antibodies: rabbit polyclonal anti-claudin 1 (1:1000 dilution, 71-7800, Invitrogen), mouse monoclonal anti-ZO-1 (1:1000 dilution, 33-9100, Thermo Fisher Scientific), mouse monoclonal anti-E cadherin (1:1000 dilution, ab231303, Abcam), Mouse monoclonal anti-GAPDH (1:1000 dilution, ab8245, Abcam) and rabbit polyclonal anti-SLC17A5 (1:1000 dilution, PA5-30517, Thermo Fisher Scientific) at 4 °C overnight. After incubation with secondary antibodies, goat anti-rabbit IgG-HRP (1:2000 dilution, SC-2004; Santa Cruz Biotechnology) or goat anti-mouse IgG-HRP (1:2000 dilution, ab205719; Abcam) at room temperature, membranes were used western ECL substrate (Bio-Rad), followed by exposure of the membranes to film and digital imaging. Image J software was used for gray scale analysis, and each independent colonic tissue lysate was technically repeated 3 times to obtain the mean value as a sample.
Fecal sample collection, intestine microbiota and metabolites assay
Feces from lower colon was obtained after the mice sacrificed, and stored at − 80 °C. Fecal DNA was extracted using a QlAamp DNA Stool Mini Kit (Qiagen, Germany). The V3–V4 region of the bacterial 16 s rRNA gene was amplified by polymerase chain reaction (PCR) using the primers 338F (5′-GTACTCCTACGGGAGGCAGCA-3′) and 806R (5′-GTGGACTACHVGGGTWTCTAAT-3′). Each sample was labeled with a unique barcode using a polymerase chain reaction (PCR). The PCR product was separated on a 2% agarose gel, and the relative concentration of the target band was measured. PCR products of equal quality from each sample according to their relative concentrations were pooled together to build a library. Sequencing was performed on an Illumina MiSeq platform.
Bioinformatics analysis
Raw paired-end reads were processed using the QIIME 2 platform (version 2020.2). Sequence quality controls were performed using DADA2: raw reads were filtered, trimmed, denoised, and dereplicated; forward and reverse sequences were merged; and chimeras were removed. Determinations of alpha and beta diversities were also conducted using QIIME 2. Alpha diversity and statistical data were calculated using the Shannon index. Beta diversity was measured using Bray–Curtis dissimilarity. Differentially abundant genera between groups were identified using linear discriminant analysis effect size (LEfSe). Predictions of the Kyoto Encyclopedia of Genes and Genomes (KEGG) metabolic pathways of the microbiota data were made using Tax4Fun.
Kaplan–Meier survial analysis
Kaplan–Meier analysis was employed to confirm the obtained results. The end of the experiment served as the cutoff point. A log rank p value of less than 0.05 was considered statistically significant.
Statistical analysis
All experiments were randomized into groups by block randomization. Data collection and analysis were performed blindly. No samples and animals were excluded from analysis. Comparisons between two groups were performed using unpaired two-tailed Student’s t tests; one-way analysis of variance (ANOVA) was used for comparisons between more than two groups. Data were expressed as the means ± standard deviation (SD). Statistical analyses and graphical generation of data were done with GraphPad Prism 9.0. Differences were considered significant at the value of P < 0.05. We used Image-J software to analyse the sum Integral optical density (IOD) of Claudin1, E cadherin, ZO-1, Sialin and MUC2 protein. We did five biological replicates for each group and three more technical replicates for each sample. We calculated the number of PCNA positive (PCNA +) per field at ×400 magnification using Image J software in six colons from six animals in each group and five fields in each tissue.
Results
Dietary nitrate promotes saliva and serum nitrate levels in aging mice
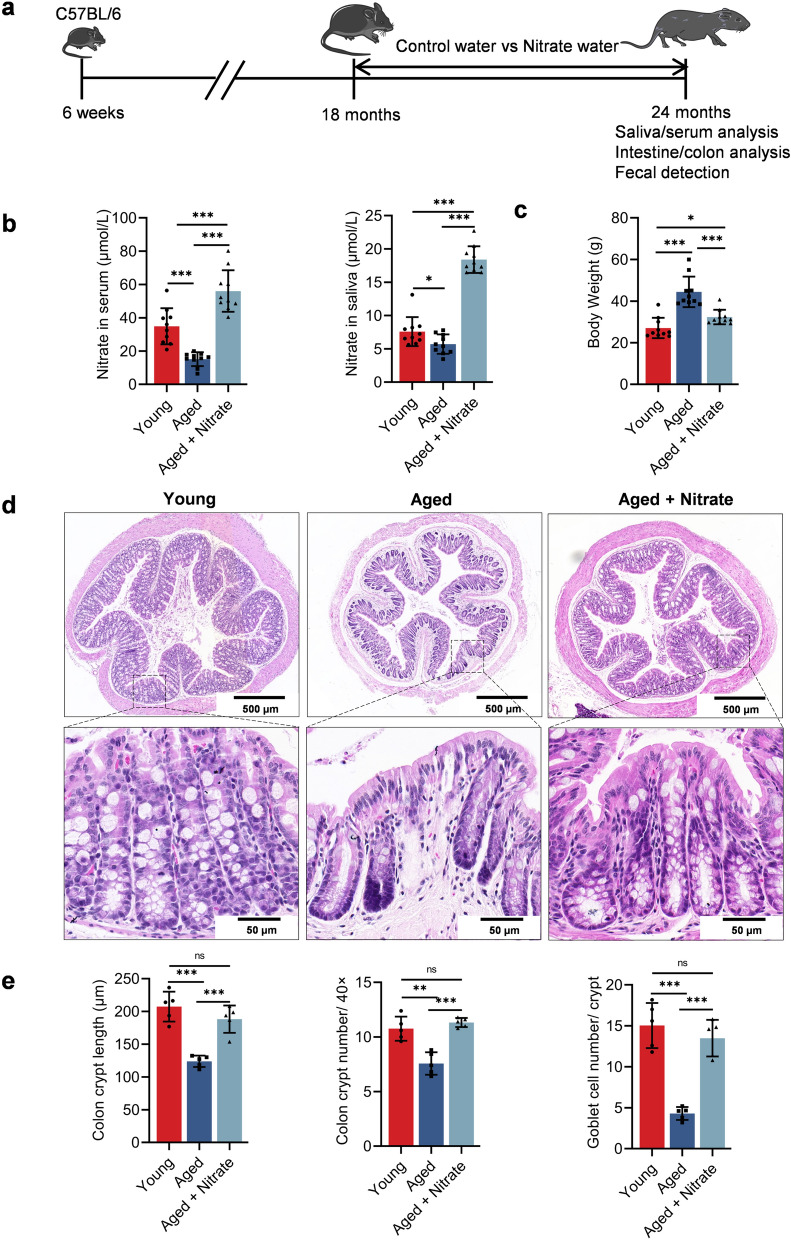
To assess the changes in the nitrate levels in aging mice, we initially examined the salivary and serum nitrate levels. Compared with the young group (8-week-old), the aged group (24-month-old mice) showed significantly and non-significantly reduced serum and salivary nitrate levels, respectively. However, long-term nitrate supplementation in the aged group (aged + nitrate group) significantly increased the salivary and serum nitrate levels (Fig. 1b). Further, long-term nitrate supplementation did not affect the mouse survival cycle throughout the observation period (Fig. S1a). Nitrate also did not alter the colon length compared to the aging group (Fig. S1b, c, d). These findings suggested that aging is associated with decreased nitrate levels in the body.
Fig. 1.
Dietary nitrate promotes salivary and serum nitrate levels and alleviates aging-related colonic frailty in aging mice. a Experiment design. Long term nitrate supplementation from 18 months of age, with regular observation until euthanasia at 24 months and subsequent histological observation. b, c Nitrate levels in saliva and serum (b) and body weight (c) of the young, aged and aged + nitrate group. d Colonic slices with HE staining were prepared for histological observation. Scale bar: 500 and 50 μm. e The quantification of crypt length, crypt number in per unit length and goblet cell number in per crypt for each group. ***Indicates significance at P < 0.001, *P < 0.05, and **P < 0.01, ns no significant. Data were shown as mean ± SD (n = 10)
Dietary nitrate reduces aged-related weight gain and improves the colon morphology
Aging is often associated with weight gain (Binyamin et al. 2020). Notably, nitrates reduced the aging-induced weight gain (Fig. 1c). Further, aging is associated with changes in the intestinal epithelial function, including intestinal inflammation, injury to the intestinal epithelial barrier, and impaired intestinal epithelial cell regeneration. Histological examination of the colon revealed that many U-shaped crypts in the young group were arranged regularly and opened directly into the intestinal cavity. The crypts were arranged closely with small spaces in between them and contained numerous goblet cells. In the aged group, the base of the crypts was thickened; further, the number and length of colonic crypts and goblet cells in per crypts was significantly decreased. In the aged + nitrate group, the number and length of colonic crypts was significantly increased, with a close arrangement and a decrease in the distance between them compared with the aged group; further, a greater increase in the number of goblet cells was observed compared to that in the aged group (Fig. 1d, e). These data suggest that nitrate ameliorates age-related decrease in colonic function.
Dietary nitrate maintains the intestinal epithelial barrier protein expression in aging mice
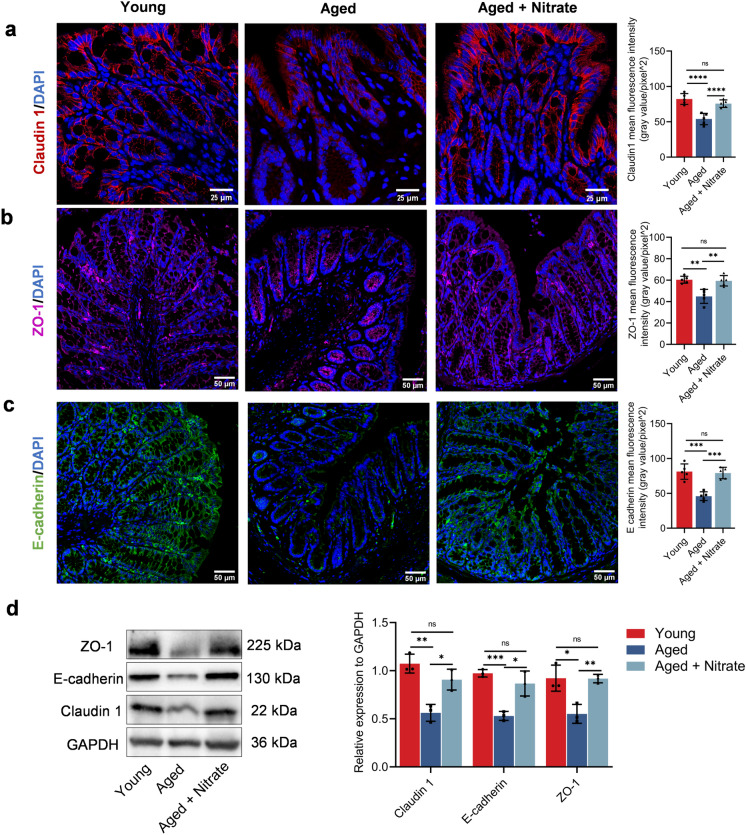
Goblet cells are responsible for maintaining the integrity of the intestinal epithelial barrier, which is formed by the epithelial cells through the protein of tight junctions (TJs) (Gustafsson and Johansson 2022). Claudin-1 is crucially involved in the formation and maintenance of TJs essential for regulating the between-cell transfer of molecules (Krug et al. 2014). In the young group, claudin 1 was mainly located between the intestinal glands and intestinal epithelial cells near the intestinal lumen. Compared with the young group, the aged group showed a decrease in the claudin-1 expression by approximately 30%, while it was recovered in the aged + nitrate group by about 92% (Fig. 2a). Zonula occludens-1 (ZO-1) is a functional protein whose distribution and expression influence the structure and function of tight junctions; additionally, it can regulate the permeability of specific ions and macromolecules near cells (Krug et al. 2014). Compared with the young group, the aged group showed decreased ZO-1 expression by approximately 24%, which was increased in the aged + nitrate group (Fig. 2b). To further identify the age-related damage to the intestinal epithelial barrier, we examined the expression levels of E-cadherin, which is an important adhesion protein that maintains intestinal barrier function. The aged group showed significantly decreased E-cadherin expression by about 50%, which was significantly increased in the aged + nitrate group (Fig. 2c). Western blotting revealed higher claudin1, ZO-1, and E-cadherin expression in the aged + nitrate group than in the aged group (Figs. 2d, S3a). Taken together, these findings indicated that dietary nitrate can effectively maintain the epithelial barrier protein levels in the aging colon.
Fig. 2.
Dietary nitrate improves the expression of intestinal epithelia barrier-related proteins in aging mice. a–c Immunofluorescence staining of Claudin 1 (red), ZO-1 (magenta), E-cadherin (green) and 4′, 6-diamidino-2-phenylindole (DAPI) (blue) in the colonic tissues in different groups. Scale bar: 25 and 50 μm. Data were shown as mean ± SD (n = 5). d Western blot analysis of claudin 1, ZO-1, E-cadherin expression in colon tissues of young, aged and nitrate (n = 3 in each group). Data were shown as mean ± SD. ****Indicates significance at P < 0.0001, ***P < 0.001, **P < 0.01, *P < 0.05, ns no significant
Dietary nitrate facilitates the proliferation of intestinal epithelial cells in aging mice
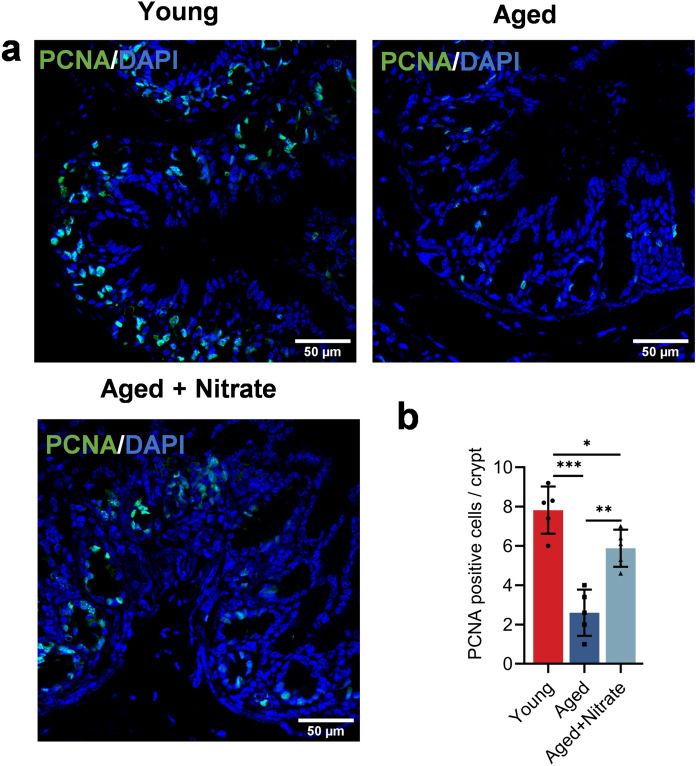
The colonic epithelium is a dynamic barrier that prevents the entry of microbes and antigens into the body. Intestinal epithelial cells proliferate in the crypts and migrate to the colon lumen, where they differentiate into cells with secretory and absorption functions to maintain epithelial homeostasis (Beumer and Clevers 2016). Proliferating cell nuclear antigen (PCNA) is a marker used for evaluating the cell proliferation status. The aged and aged + nitrate group showed a sharp decrease and significant decrease in the PCNA-positive cells, respectively (Fig. 3a). Taken together, these results indicated that dietary nitrate can effectively promote aged mice intestinal epithelial proliferation.
Fig. 3.
Dietary nitrate facilitates the proliferation of intestinal epiyhelial cells in aging mice. a, b Immunofluorescence staining of PCNA (green) and DAPI (blue) in the colonic tissues in different groups. Scale bar: 50 μm. Data were shown as mean ± SD (n = 5). ***Indicates significance at P < 0.001, **P < 0.01, *P < 0.05, ns no significant
Dietary nitrate promotes the Sialin expression in the colon epithelial cells of aging mice
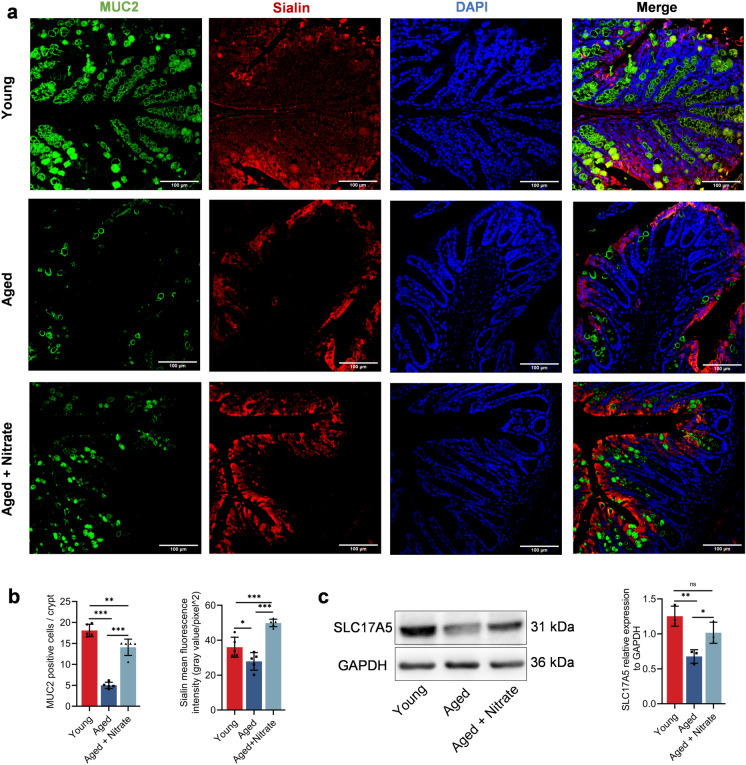
Dietary nitrate stably exists and exerts a protective effect in the gastrointestinal tract (Lundberg et al. 2008). Sialin facilitates the entry of nitrate into the cells of mammals (Qu et al. 2016; Qin et al. 2012). The interaction between nitrate and Sialin is crucial for the maintaining of salivary gland homeostasis. Mucin-2 (MUC2), which is the main component of the intestinal mucus layer, covers the top of the intestinal epithelial cells; further, it is mainly secreted by goblet cells. Muc2 is crucially involved in lubricating the intestine, providing adhesion sites for intestinal antibacterial proteins and symbiotic bacteria, and preventing the invasion of intestinal pathogens and harmful substances (Luis and Hansson 2023). To investigate how nitrate maintains the integrity of the intestinal epithelial barrier, we examined the changes in Sialin expression in the colon epithelial cells. The aged group showed sharply reduced colonic MUC2 expression, which was significantly improved in the aged + nitrate group (Fig. 4a). Compared with the young group, the aged group showed a decrease in the number of MUC2 positive cells by approximately 70%, which was significantly increased in the aged + nitrate group (Fig. 4b). The trend of Sialin protein was consistent with that of MUC2 in the aged + nitrate group (Figs. 4a–c, S3b). These results suggest that nitrate restored the age-related decrease in the MUC2 expression and Sialin protein in the colonic epithelial cell was associated with the decreased of MUC2 protein levels in the aged mice.
Fig. 4.
Dietary nitrate promotes the Sialin expression in colon epithelial cells of aging mice. a Immunofluorescence staining of MUC2 (green), Sialin (red) and DAPI (blue) in the colonic tissues in the group of young, aged and nitrate. Scale bar: 100 μm. b Quantification of the relative area of MUC2 and Sialin, n = 5 in each group. c Western blot analysis of Sialin expression in colon tissues of young, aged and nitrate, n = 3 per group. ***Indicates significance at P < 0.001, **P < 0.01. Data were shown as mean ± SD
Dietary nitrate regulates the composition and production of short-chain fatty acids in aging mice
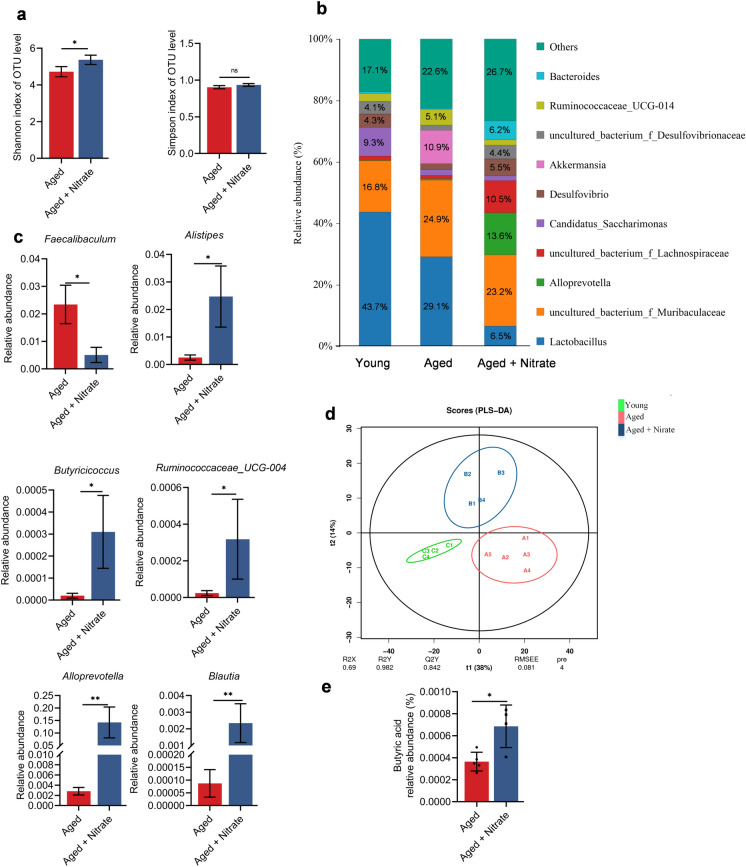
Intestinal microbiota homeostasis is closely related to the maintenance of the intestinal epithelial barrier function. The intestinal microbiota produces several metabolites, including short-chain fatty acids (SCFAs), bile acids, and lipopolysaccharides, which affect the intestinal environment, intestinal epithelial barrier, and other physiological functions (Portincasa et al. 2022). Damage to the intestinal epithelial barrier can disturb the intestine microbiota and further aggravate the body damage. Therefore, 16 s rRNA gene sequencing was used to explore the effects of nitrate on the intestinal microbiota of aging mice. Compared with the aged group, the aged + nitrate group had a higher index of Chao1, Ace, and Shannon, with no significant difference in the Simpson index (Figs. 5a, S2a–d). This suggested increased species abundance and diversity in the aged + nitrate group. At the family level, Erysipelotrichaceae, which is positively correlated with aging, increased and decreased in abundance in the aged and aged + nitrate groups, respectively. The abundance of beneficial bacteria, such as Lachnospiraceae, Bacteroidaceae, and Prevotellaceae, increased in the aged + nitrate group (Fig. S2e). Bacteria with the highest abundances at the genus level were Lactobacillus, Muribaculaceae, Alloprecotella, Lachnospiraceae, Candidatus_Saccharimonas, Desulfovibrio, Akkermansia, Desulfovibrionaceae, Ruminococcaceae_UGG-014 and Bacteroides (Fig. 5b). Furthermore, the genus-level analysis showed that nitrate supplementation increased the abundance of Alistipes, Blautia, Alloprevotella, Butyricicoccus, and Ruminococcaceae in the colon of aging mice. The abundance of Faecalibaculum, which is associated with aging, was decreased in the aged + nitrate group (Fig. 5c). The intestinal microbiota maintains intestinal homeostasis by producing bioactive metabolites, with the colon being the main site of microbial fermentation (Portincasa et al. 2022). We observed significant among-group differences in the colon metabolite levels (Fig. 5d). SCFAs are crucially involved in the maintenance of intestinal epithelial homeostasis. Specifically, the abundance of butyric acid was decreased and increased in the aged and aged + nitrate groups, respectively (Fig. 5e). Taken together, nitrate supplementation changed the bacteria composition in colon tissue of aging mice, and increased the abundance of some beneficial bacterium and increased the butyric acid content in the colon.
Fig. 5.
Dietary nitrate regulates the composition of the intestinal microbiota and promotes SCFAs production in aging mice. a–c Intestinal microbiota analysis of young (n = 4), aged (n = 5) and nitrate (n = 4). a Comparison of alpha diversity of the colonic microbiota using Shannon and Simpson index. b Average relative abundance of colonic microbiota at genus level in different groups. c The different microbiota in the colonic at genus level. d The difference of intestinal metabolites in three groups was analyzed by partial least squares discriminant analysis (PLA-DS). e Concentrations of butyric acid in aged and nitrate group. **Indicates significance at P < 0.01, *P < 0.05, ns no significant. Data were shown as mean ± SD
Sialin maintains colon epithelial barrier-related proteins and the proliferation of colon epithelial cells
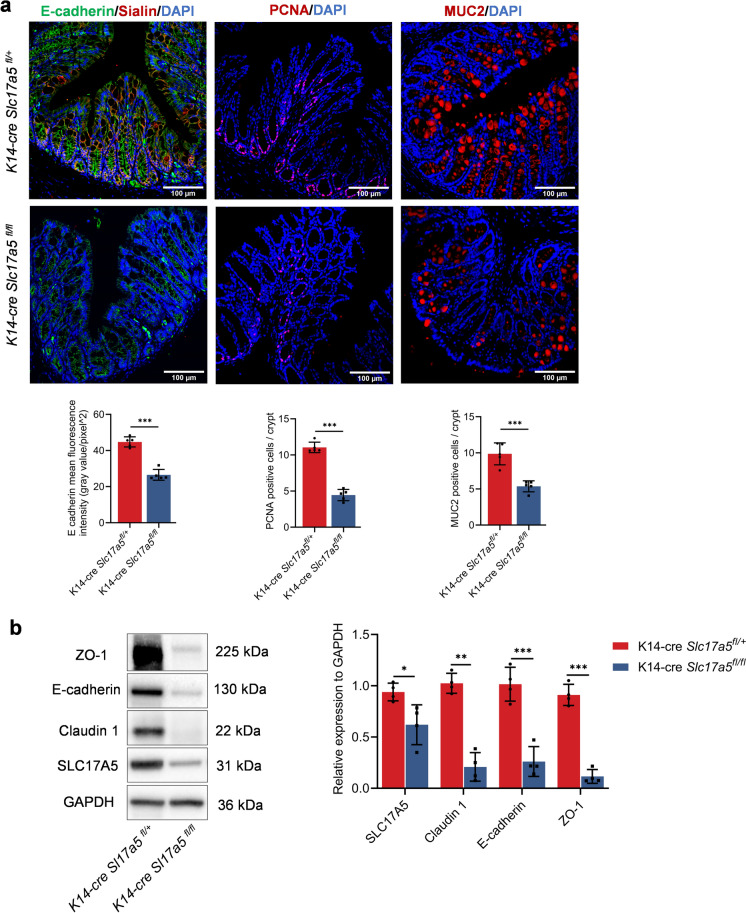
Studies have shown that Sialin is an important protein that transports nitrate and is expressed by multiple organs of the body. The nitrate-Sialin feedback loop plays an important role in the regulation of salivary gland epithelial homeostasis after irradiation (Wang and Qin 2022; Feng et al. 2021). In addition, Sialin in the colonic epithelial cell was associated with nitrate protection against aging-related colonic injury. We examined the expression of proteins that maintain the integrity of the colonic epithelial barrier in conditioned Slc17a5-knockout mice. Accordingly, compared with K14-Cre; Slc17a5fl/+mice, the K14-Cre; Slc17a5fl/fl knockout mice showed significantly decreased proportions of E-cadherin, colonic proliferative cells (PCNA+), MUC2 positive cells in per crypts (Figs. 6a, b, S3c). These results suggest that conditional Slc17a5 knockout directly affected colonic epithelial homeostasis.
Fig. 6.
Sialin maintains colon epithelial barrier-related proteins and the proliferation of colon epithelial cells. a Immunofluorescence staining of E-cadherin (green), Sialin (red), PCNA (red), MUC2 (red) and DAPI (blue) in the colonic tissues in the group of K14-cre; Slc17a5fl/+ control and K14-cre; Slc17a5fl/fl mice. Scale bar: 100 μm. Data were shown as mean ± SD (n = 5 per group). ***Indicates significance at P < 0.001, *P < 0.05. b Western blot analysis of ZO-1, E-cadherin, Claudin 1 and Sialin expression in colon tissues of K14-cre; Slc17a5fl/+ and K14-cre; Slc17a5fl/fl mice (n = 5 per group). Data were shown as mean ± SD
Discussion
Our findings revealed a significant increase in the salivary nitrate levels during aging. Nitrate enhances the proliferation of intestinal epithelial cells by upregulating Sialin expression, which maintains the epithelial barrier protein levels in the aging mice. Dietary nitrate also changed aging-related intestinal microbial composition and promotes butyric acid production in the aged colon tissue. These results underscore the crucial role of nitrate-Sialin in maintaining aging-associated colonic epithelial homeostasis.
Nitrate has therapeutic and preventive efficacy in intestinal microbiota dysbiosis and aging-related diseases (Hu et al. 2020; Wang et al. 2020a, b; Wang et al. 2018). Long-term dietary nitrate supplementation has recently been shown to promote vascular function and improve health in aged individuals (Carvalho et al. 2021). In our study, nitrate supplementation increased the salivary and serum nitrate levels in aged mice; further, it mitigated aging-related body weight gain. Nitrate promoted the number of colonic goblet cells and crypts in the aged mice. Goblet cells and intestinal epithelial cells form a mechanical barrier in the colonic upper layer via TJs proteins (Krug et al. 2014). The colonic epithelial barrier is crucially involved in maintaining the health by allowing absorption of essential nutrients as well as preventing the entry of microorganisms and antigens into the body (Parrish 2017). Damage to the intestinal epithelial barrier can cause “intestinal leakage”, resulting in increased intestinal epithelial permeability, which in turn causes inflammation (Wang and Yang 2024; Tran and Greenwood-Van 2013; Okumura and Takeda 2017). Decreased expression of claudin 1, ZO-1, and E-cadherin, which maintain epithelial barrier function, was observed in the aging group. These results indicate that aging impaired the colon barrier function and increased epithelial permeability, which is consistent with previous results (Parrish 2017). Nitrate significantly increased the expression of the three aforementioned functional proteins. MUC2 is mainly secreted by goblet cells and is crucially involved in maintaining the intestinal mucus barrier (Portincasa et al. 2022). The presence of intestinal MUC2 is of great value in maintaining the stability of the intestinal microenvironment and normal function of intestinal epithelial cells. We found that nitrate restored the age-related decrease in the MUC2 expression. This suggests that nitrate may play a crucial role in reversing aging-related impairment of the colon barrier function.
The colonic epithelium is a dynamic barrier; the continuous regeneration of intestinal epithelial cells along the crypts is a sign of maintaining intestinal homeostasis (Okumura and Takeda 2017; Beumer and Clevers 2016). In our study, the aging group showed a significantly decreased number of proliferating intestinal epithelial cells in the colon, which is consistent with previous studies (Jasper 2020). Our findings revealed a significantly increased number of proliferating intestinal epithelial cells with nitrate supplementation. These data indicating that nitrate maintains colon epithelial homeostasis by promoting intestinal epithelial cell proliferation. The proliferation and differentiation of intestinal epithelial cells are coordinated and controlled by the epithelium and stroma through various signaling molecules. Recent studies have shown that intestinal microbiota can directly or indirectly communicate with the intestinal epithelial cells to regulate their proliferation and differentiation (Hou et al. 2017). Based on the analysis of the intestinal microbiota changes, the nitrate group changed the bacteria composition in colon tissue of aging mice. Through the analysis of the relative abundance composition of the top ten bacteria among each group, the nitrate group had a decreased relative proportion of Lactobacullus at the genus level compared with the aged group, surprisingly. Lactobacillus is a very important beneficial bacteria, which plays an important role in maintaining the balance of intestinal microbiota, improving digestive function and enhancing immunity (Chuang et al. 2024). We hypothesized that the decrease in the percentage of genus-level Lactobacillus could be due to two reasons: (i) nitrate affects lactic acid metabolism, and (ii) an elevated percentage of the remaining beneficial bacteria, e.g., Lachnospiraceae, Alloprecotella and Bacteroides leads to a decrease in the relative percentage of Lactobacillus. Moreover, a decreased abundance of Blautia, Alloprevotella, Butyricicoccus, and Ruminococcaceae was observed in the colon of aging mice, which was increased by nitrate supplementation. The study found that these bacteria were associated with SCFAs production (Zhuang et al. 2021; Liu et al. 2021; Xie et al. 2022). SCFAs are crucially involved in the maintenance of intestinal epithelial homeostasis. Subsequently, we performed a non-targeted metabolomics analysis of the microbial metabolites to screen for the differential metabolite, butyric acid. The amount of butyric acid was decreased in the aging mice, and increased after nitrate intake. Studies have found that when the intestinal epithelial barrier is damaged, intestinal stem cells located in the crypt directly contact the butyric acid, which inhibits their proliferation through the transcription factor Foxo3 (Kaiko et al. 2016). Intestinal epithelial cells are protected by the intestinal crypts to avoid contact with butyric acid, which maintains stem cell activity. Intestinal epithelial barrier dysfunction often leads to decreased butyrate synthesis, which can promote intestinal barrier function and accelerate the repair of intestinal epithelial cells by regulating the expression of synaptopodin (an F-actin-binding protein) (Wang et al. 2020a, b). Therefore, intestinal epithelial permeability may increase following aging-related intestinal function impairment; furthermore, direct contact between butyric acid and the intestinal epithelial cells in the intestinal crypt inhibits their proliferation. In contrast, nitrate promotes the production of butyric acid by increasing the abundance of butyric bacteria, which maintains intestinal homeostasis. Further studies are warranted to elucidate specific mechanisms through which nitrate and butyric acid maintain intestinal homeostasis.
To further examine the mechanism through which nitrate maintains colon epithelial function, we conducted a localization analysis of Sialin in the colon tissues and found that Sialin was mainly located in the colon epithelial cells, with small amounts in the goblet cells. Nitrate significantly promotes Sialin expression in the aged colonic tissues. However, the mechanism that how nitrate maintains age-related colon epithelial homeostasis through Sialin remains unclear. We subsequently blocked Sialin expression by conditionally knocking out Slc17a5 and observed changes in the colonic epithelial barrier. We found that the epithelial barrier-related proteins were decreased in the knockout mice; further, decreased proliferation of the epithelial cells and number of MUC2-positive cells in the colon was observed, which affects colon epithelial homeostasis. Taken together, Sialin-mediated nitrate transportation may a key pathway for nitrate to maintain the homeostasis in the aging intestinal epithelium, and thus may guide dietary interventions in aging. However, specific mechanism of activation of the target cell of Sialin warrants further study.
In the field of ageing research, there are still seven knowledge gaps that urgently need to be addressed, mainly in the evolution of longevity, biological survival and death, and heterogeneity of the aging phenotype (Rattan 2024). Identifying the harmful, beneficial, and neutral changes that occur during aging is a critical first step in maintaining and enhancing health. Consistent with previous studies, our research found that intestinal epithelial cell dyshomeostasis can serve as a valuable indicator of the aging process. Reduced expression of intestinal epithelial barrier-related proteins, slowed proliferation and renewal of epithelial cells, and the change of intestinal bacterias composition can provide evaluate the health status during the aging process. Here, nitrates serves a new potential therapeutic approaches to alleviating aged-related intestinal dyshomeostasis. However, our study has some limitations. We focused only on intestinal alterations. In addition, it is unclear how nitrate regulates epithelial cell proliferation after entering the colon. Further research is needed to elucidate the exact mechanism by which nitrate maintains ageing colonic homeostasis.
In conclusion, the reduction of the nitrate levels was associated with the decreased expression of age-related colonic epithelial barrier proteins. The effect of nitrate on the aged intestinal homeostasis was largely attributed to the increased expression of Sialin protein. Our study provides new evidence for the crucial role of nitrate in aging and will aid in potential approaches for age-related diseases.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by the Beijing Municipal Government grant (Beijing Laboratory of Oral Health, PXM2021-014226-000041), the Beijing Municipal Science and Technology Commission (Z181100001718208), the Beijing Municipal Education Commission (119207020201), the Innovation Research Team Project of Beijing Stomatological Hospital, Capital Medical University (CXTD202201), the Chinese Research Unit of Tooth Development and Regeneration, Academy of Medical Sciences (2019-12M-5-031), the National Natural Science Foundation of China (92049201, 82030031, 81991504, 92149301, L2224038, and 82001067), the Beijing Advanced Innovation Center for Big Data-based Precision Medicine (PXM2021_014226_000026), the Beijing Municipal Government (Beijing Scholar Program, PXM2020_014226_000005 and PXM2021_014226_000020), the Beijing Municipal Colleges and Universities High Level Talents Introduction and Cultivate Project-Beijing Great Wall Scholar Program (CIT&TCD 20180332), and the National Key Research and development Program (2022YFA1104401). Scientific Research Common Program of Beijing Municipal Commission of Education (KM202110025009) and Beijing Municipal Natural Science Foundation (7232071). State Key Laboratory of Oral Diseases Open Fund (SKLOD2024OF15). Young Scientist Program of Beijing Stomatological Hospital, Capital Medical University (NO.YSP202205).
Author contributions
X W performed the experiments and wrote the manuscript. H L, YM W and JS W aided in the process of experiments and collected the samples. X W, H L and CM Z analyzed the data and reviewed the manuscript. S W, and L H designed and supervised the project. All authors approved the final version of the manuscript.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Songlin Wang, Email: slwang@ccmu.edu.cn.
Lei Hu, Email: hulei@ccmu.edu.cn.
References
- An R, Wilms E, Masclee AAM, Smidt H, Zoetendal EG, Jonkers D (2018) Age-dependent changes in GI physiology and microbiota: time to reconsider? Gut 67(12):2213–2222. 10.1136/gutjnl-2017-315542 [DOI] [PubMed] [Google Scholar]
- Beumer J, Clevers H (2016) Regulation and plasticity of intestinal stem cells during homeostasis and regeneration. Development 143(20):3639–3649. 10.1242/dev.133132 [DOI] [PubMed] [Google Scholar]
- Binyamin D, Werbner N, Nuriel-Ohayon M, Uzan A, Mor H, Abbas A, Ziv O, Teperino R, Gutman R, Koren O (2020) The aging mouse microbiome has obesogenic characteristics. Genome Med 12(1):87. 10.1186/s13073-020-00784-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandhorst S, Choi IY, Wei M, Cheng CW, Sedrakyan S, Navarrete G, Dubeau L, Yap LP, Park R, Vinciguerra M, Di Biase S, Mirzaei H, Mirisola MG, Childress P, Ji LY, Groshen S, Penna F, Odetti P, Perin L, Conti PS, Longo VD (2015) A periodic diet that mimics fasting promotes multi-system regeneration, enhanced cognitive performance, and healthspan. Cell Metab 22(1):86–99. 10.1016/j.cmet.2015.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buford TW (2017) (Dis)Trust your gut: the gut microbiome in age-related inflammation, health, and disease. Microbiome 5(1):80. 10.1186/s40168-017-0296-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho LRRA, Guimarães DD, Flôr AFL, Leite EG, Ruiz CR, de Andrade JT, Monteiro MMO, Balarini CM, Lucena RB, Sandrim VC, Lundberg JO, Weitzberg E, Carlström M, Braga VA (2021) Effects of chronic dietary nitrate supplementation on longevity, vascular function and cancer incidence in rats. Redox Biol 48:102209. 10.1016/j.redox.2021.102209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang YF, Fan KC, Su YY, Wu MF, Chiu YL, Liu YC, Lin CC (2024) Precision probiotics supplement strategy in aging population based on gut microbiome composition. Briefings Bioinform 25(4):bbae351. 10.1093/bib/bbae351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duregon E, Fernandez ME, Martinez Romero J, Di Germanio C, Cabassa M, Voloshchuk R, Ehrlich-Mora MR, Moats JM, Wong S, Bosompra O, Rudderow A, Morrell CH, Camandola S, Price NL, Aon MA, Bernier M, de Cabo R (2023) Prolonged fasting times reap greater geroprotective effects when combined with caloric restriction in adult female mice. Cell Metab 35(7):1179–1194. 10.1016/j.cmet.2023.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng XY, Wu ZF, Xu JJ, Xu YP, Zhao B, Pang BX, Qu XM, Hu L, Hu L, Fan ZP, Jin LY, Xia DS, Chang SM, Wang JS, Zhang CM, Wang SL (2021) Dietary nitrate supplementation prevents radiotherapy-induced xerostomia. Elife 10:e70710. 10.7554/eLife.70710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk MC, Zhou J, Boutros M (2020) Ageing, metabolism and the intestine. EMBO Rep 21(7):e50047. 10.15252/embr.202050047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill SK, Rossi M, Bajka B, Whelan K (2021) Dietary fibre in gastrointestinal health and disease. Nat Rev Gastroenterol Hepatol 18(2):101–116. 10.1038/s41575-020-00375-4 [DOI] [PubMed] [Google Scholar]
- Gustafsson JK, Johansson MEV (2022) The role of goblet cells and mucus in intestinal homeostasis. Nat Rev Gastroenterol Hepatol 19(12):785–803. 10.1038/s41575-022-00675-x [DOI] [PubMed] [Google Scholar]
- Haran JP, McCormick BA (2021) Aging, frailty, and the microbiome-how dysbiosis influences human aging and disease. Gastroenterology 160(2):507–523. 10.1053/j.gastro.2020.09.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou QH, Ye LL, Huang LL, Yu QH (2017) The research progress on intestinal stem cells and its relationship with intestinal microbiota. Front Immunol 8:599. 10.3389/fimmu.2017.00599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L, Jin LY, Xia DS, Zhang Q, Ma LS, Zheng H, Xu TS, Chang SM, Li XC, Xun Z, Xu YP, Zhang CM, Chen F, Wang SL (2020) Nitrate ameliorates dextran sodium sulfate-induced colitis by regulating the homeostasis of the intestinal microbiota. Free Radic Biol Med 152:609–621. 10.1016/j.freeradbiomed.2019.12.002 [DOI] [PubMed] [Google Scholar]
- Jasper H (2020) Intestinal stem cell aging: origins and interventions. Annu Rev Physiol 82:203–226. 10.1146/annurev-physiol-021119-034359 [DOI] [PubMed] [Google Scholar]
- Jin LY, Qin LZ, Xia DS, Liu XB, Fan ZP, Zhang CM, Gu LK, He JQ, Ambudkar IS, Deng DJ, Wang SL (2013) Active secretion and protective effect of salivary nitrate against stress in human volunteers and rats. Free Radic Biol Med 57:61–67. 10.1016/j.freeradbiomed.2012.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AM, Thompson C, Wylie LJ, Vanhatalo A (2018) Dietary nitrate and physical performance. Annu Rev Nutr 38:303–328. 10.1146/annurev-nutr-082117-051622 [DOI] [PubMed] [Google Scholar]
- Kaiko GE, Ryu SH, Koues OI, Collins PL, Solnica-Krezel L, Pearce EJ, Pearce EL, Oltz EM, Stappenbeck TS (2016) The colonic crypt protects stem cells from microbiota-derived metabolites. Cell 165(7):1708–1720. 10.1016/j.cell.2016.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keebaugh ES, Ja WW (2017) Breaking down walls: microbiota and the aging gut. Cell Host Microbe 21(4):417–418. 10.1016/j.chom.2017.03.013 [DOI] [PubMed] [Google Scholar]
- Khorasani V, Jeddi S, Yaghmaei P, Tohidi M, Ghasemi A (2019) Effect of long-term sodium nitrate administration on diabetes-induced anemia and glucose homeostasis in obese type 2 diabetic male rats. Nitric Oxide 86:21–30. 10.1016/j.niox.2019.02.003 [DOI] [PubMed] [Google Scholar]
- Krug SM, Schulzke JD, Fromm M (2014) Tight junction, selective permeability, and related diseases. Semin Cell Dev Biol 36:166–176. 10.1016/j.semcdb.2014.09.002 [DOI] [PubMed] [Google Scholar]
- Liu XM, Mao BY, Gu JY, Wu JY, Cui SM, Wang G, Zhao JX, Zhang H, Chen W (2021) Blautia—a new functional genus with potential probiotic properties? Gut Microbes 13(1):1–21. 10.1080/19490976.2021.1875796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luis AS, Hansson GC (2023) Intestinal mucus and their glycans: a habitat for thriving microbiota. Cell Host Microbe 31(7):1087–1100. 10.1016/j.chom.2023.05.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg JO, Weitzberg E, Gladwin MT (2008) The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov 7(2):156–167. 10.1038/nrd2466 [DOI] [PubMed] [Google Scholar]
- Lundberg JO, Carlström M, Weitzberg E (2018) Metabolic effects of dietary nitrate in health and disease. Cell Metab 28(1):9–22. 10.1016/j.cmet.2018.06.007 [DOI] [PubMed] [Google Scholar]
- O’Toole PW, Jeffery IB (2015) Gut microbiota and aging. Science 350(6265):1214–1215. 10.1126/science.aac8469 [DOI] [PubMed] [Google Scholar]
- O’Toole PW, Jeffery IB (2018) Microbiome-health interactions in older people. Cell Mol Life Sci 75(1):119–128. 10.1007/s00018-017-2673-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumura R, Takeda K (2017) Roles of intestinal epithelial cells in the maintenance of gut homeostasis. Exp Mol Med 49(5):e338. 10.1038/emm.2017.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish AR (2017) The impact of aging on epithelial barriers. Tissue Barriers 5(4):e1343172. 10.1080/21688370.2017.1343172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portincasa P, Bonfrate L, Vacca M, De Angelis M, Farella I, Lanza E, Khalil M, Wang DQ, Sperandio M, Di Ciaula A (2022) Gut microbiota and short chain fatty acids: implications in glucose homeostasis. Int J Mol Sci 23(3):1105. 10.3390/ijms23031105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin LZ, Liu XB, Sun QF, Fan ZP, Xia DS, Ding G, Ong HL, Adams D, Gahl WA, Zheng CY, Qi SR, Jin LY, Zhang CM, Gu LK, He JQ, Deng DJ, Ambudkar IS, Wang SL (2012) Sialin (SLC17A5) functions as a nitrate transporter in the plasma membrane. Proc Natl Acad Sci USA 109(33):13434–13439. 10.1073/pnas.1116633109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu XM, Wu ZF, Pang BX, Jin LY, Qin LZ, Wang SL (2016) From nitrate to nitric oxide: the role of salivary glands and oral bacteria. J Dent Res 95(13):1452–1456. 10.1177/0022034516673019 [DOI] [PubMed] [Google Scholar]
- Rattan SIS (2024) Seven knowledge gaps in modern biogerontology. Biogerontology 25(1):1–8. 10.1007/s10522-023-10089-0 [DOI] [PubMed] [Google Scholar]
- Sbierski-Kind J, Grenkowitz S, Schlickeiser S, Sandforth A, Friedrich M, Kunkel D, Glauben R, Brachs S, Mai K, Thürmer A, Radonić A, Drechsel O, Turnbaugh PJ, Bisanz JE, Volk HD, Spranger J, von Schwartzenberg RJ (2022) Effects of caloric restriction on the gut microbiome are linked with immune senescence. Microbiome 10(1):57. 10.1186/s40168-022-01249-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran L, Greenwood-Van Meerveld B (2013) Age-associated remodeling of the intestinal epithelial barrier. J Gerontol A Biol Sci Med Sci 68(9):1045–1056. 10.1093/gerona/glt106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SL, Qin LZ (2022) Homeostatic medicine: a strategy for exploring health and disease. Curr Med (cham) 1(1):16. 10.1007/s44194-022-00016-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SJ, Yang H (2024) Low-molecular-weight heparin ameliorates intestinal barrier dysfunction in aged male rats via protection of tight junction proteins. Biogerontology. 10.1007/s10522-024-10118-6 [DOI] [PubMed] [Google Scholar]
- Wang HF, Hu L, Li L, Wu XS, Fan ZP, Zhang CM, Wang JS, Jia JD, Wang SL (2018) Inorganic nitrate alleviates the senescence-related decline in liver function. Sci China Life Sci 61(1):24–34. 10.1007/s11427-017-9207-x [DOI] [PubMed] [Google Scholar]
- Wang RX, Lee JS, Campbell EL, Colgan SP (2020a) Microbiota-derived butyrate dynamically regulates intestinal homeostasis through regulation of actin-associated protein synaptopodin. Proc Natl Acad Sci USA 117(21):11648–11657. 10.1073/pnas.1917597117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WL, Hu L, Chang SM, Ma LS, Li XC, Yang Z, Du CL, Qu XM, Zhang CM, Wang SL (2020b) Total body irradiation-induced colon damage is prevented by nitrate-mediated suppression of oxidative stress and homeostasis of the gut microbiome. Nitric Oxide Biol Chem 102:1–11. 10.1016/j.niox.2020.05.002 [DOI] [PubMed] [Google Scholar]
- Xia DS, Deng DJ, Wang SL (2003) Destruction of parotid glands affects nitrate and nitrite metabolism. J Dent Res 82(2):101–105. 10.1177/154405910308200205 [DOI] [PubMed] [Google Scholar]
- Xie J, Li LF, Dai TY, Qi X, Wang Y, Zheng TZ, Gao XY, Zhang YJ, Ai Y, Ma L, Chang SL, Luo FX, Tian Y, Sheng J (2022) Short-chain fatty acids produced by Ruminococcaceae mediate α-Linolenic acid promote intestinal stem cells proliferation. Mol Nutr Food Res 66(1):e2100408. 10.1002/mnfr.202100408 [DOI] [PubMed] [Google Scholar]
- Xu YF, Sa YQ, Zhang CM, Wang JS, Shao QQ, Liu J, Wang SL, Zhou J (2024) A preventative role of nitrate for hypoxia-induced intestinal injury. Free Radic Biol Med 213:457–469. 10.1016/j.freeradbiomed.2024.01.030 [DOI] [PubMed] [Google Scholar]
- Yousefzadeh N, Jeddi S, Zarkesh M, Kashfi K, Ghasemi A (2023) Altered sialin mRNA gene expression in type 2 diabetic male Wistar rats: implications for nitric oxide deficiency. Sci Rep 13(1):4013. 10.1038/s41598-023-31240-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapata HJ, Quagliarello VJ (2015) The microbiota and microbiome in aging: potential implications in health and age-related diseases. J Am Geriatr Soc 63(4):776–781. 10.1111/jgs.13310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang P, Li HY, Jia W, Shou QY, Zhu Y, Mao L, Wang WQ, Wu F, Chen XQ, Wan XZ, Wu YQ, Liu XH, Li Y, Zhu FH, He LL, Chen JN, Zhang Y, Jiao JJ (2021) Eicosapentaenoic and docosahexaenoic acids attenuate hyperglycemia through the microbiome-gut-organs axis in db/db mice. Microbiome 9(1):185. 10.1186/s40168-021-01126-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.