Abstract
Aim
Postoperative gastrointestinal dysfunction (POGD) is a common complication following colorectal surgery. This study aimed to investigate the incidence and risk factors of POGD after minimally invasive surgery and to assess the relationship between robotic surgery, POGD, and their outcomes.
Method
Patients who had undergone minimally invasive colorectal surgery at our institution between July 2018 and November 2023 were retrospectively enrolled. POGD was diagnosed based on the presence of two or more intestinal symptoms within 72 h or more after surgery. Risk factors were identified through regression analyses, and the impact of POGD on outcomes was assessed using linear regression.The association between those factors was assessed using subgroup analysis and hierarchical regression.
Results
A total of 226 patients were included in the analysis, including 33 with POGD. POGD occurred in 14.6% of patients, with a lower incidence in robotic surgery (7.3%) than in laparoscopic surgery (19.8%). Multivariate analysis indicated that robotic surgery had a protective effect, while blood loss exceeding 50 ml was an independent risk factor for POGD. POGD was also correlated with longer length of stays and higher costs. The association between POGD, length of stay, and cost varied depending on the surgical platform. Robotic surgery exacerbated the effect of POGD on short-term outcomes, which aligned with the observed significant interaction effect.
Conclusion
POGD remains a prevalent postoperative disease. Preventive strategies, including meticulous hemostasis techniques and robotic surgery, should be prioritized by healthcare professionals to reduce POGD risk, improve short-term outcomes, and preserve healthcare resources.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00384-024-04733-5.
Keywords: Colorectal cancer, POGD, Minimally invasive surgery, Risk factors, Clinical impact
Introduction
Postoperative gastrointestinalf dysfunction (POGD) is a common complication of colorectal surgery,incidence ranging from 4.5% to 42.7% [1–6]. Risk factors of POGD include sex, smoking, chronic obstructive pulmonary disease (COPD), and previous abdominal surgery [2–4]. Patients with POGD are at an elevated risk of experiencing other postoperative complications, leading to greater expenses [7, 8]. Therefore, prioritizing reduction of postoperative ileus is essential to reduce healthcare costs and improve patient outcomes.
However, the lack of standardized reporting methods does not ensure accurate and comparable data across previous studies. Pozios et al. [1] defined POGD as the continued use of a gastric tube, vomiting, or cessation of bowel movements after the third day of surgery, while Millan et al. [2] defined it as the lack of flatus on the sixth day after surgery. Quiroga-Centeno et al. [6] defined POGD as a condition where a patient does not have bowel movements or exhaust for more than 3 days after surgery, and also experiences bloating and lack of bowel sounds.
Although minimally invasive surgery has reduced the incidence of POGD, it remains a prevalent issue [9]. Identifying the independent factors influencing POGD in minimally invasive colorectal surgery is essential to optimizing patient recovery and outcomes. The advantages of robotic surgery over laparoscopic surgery in terms of surgical efficacy and mortality have been reported [10]. However, the impact of robotic surgery on POGD and its association with length of stay and cost remains limited.
The primary objective of this study was to determine the impact of robotic surgery on the incidence and risk factors of POGD after minimally invasive colorectal surgery. The secondary objectives included evaluating the relationship between robotic surgery, POGD, and short-term outcomes.
Methods
This retrospective analysis included the clinical data of patients who underwent robotic or laparoscopic colorectal surgery between July 1, 2018 and November 30, 2023 at the Fourth Affiliated Hospital of Guangxi Medical University. The study included patients who met the following inclusion criteria: (I) pathologically proven colorectal adenocarcinoma, (II) simultaneous resection of the primary tumor and metastatic lymph node for curative purposes, and (III) complete medical records. Patients with bleeding disorders, connective tissue disease, mechanical obstruction, slow-transmitting constipation, or an unresectable primary tumor were excluded from the study. To clarify the role of robotic surgery in minimally invasive surgery, we selected laparoscopic surgery during the same period as the control group. The study was approved by the Ethics Committee of the Fourth Affiliated Hospital of Guangxi Medical University (no. KY2022260). The participants were informed that their perioperative clinical data would be retrospectively analyzed and published. All analyses were conducted after patient de-identification, ensuring that there was no interference with therapeutic management.
The demographic and clinicopathological characteristics, medical treatment history, and oncological results were reviewed using an electronic medical record system. POGD was defined as an the I-FEED score of ≥ 6, according to the American Society for Enhanced Recovery (ASER) and the Perioperative Quality Initiative (POQI) Joint Consensus Statement [11]. This criterion identifies POGD based on the presence of two or more symptoms occurring within 2 days or longer after surgery, including the complete inability to ingest orally, persistent nausea, abdominal distension, abdominal percussion drumming, and persistent nausea accompanied by severe vomiting or biliary vomiting. For anastomosis techniques, we categorized them based on the intestinal structures used for gastrointestinal reconstruction: ileo-colic, colo-colic, and colo-rectal anastomoses. ERAS (Enhanced Recovery After Surgery) was defined according to the guidelines established by the ERAS Society [12]. Additional organ resection was defined as the excision of tissue from adjacent organs, with or without involving multiple organ systems. Totally laparoscopic surgery was defined as a procedure where all anastomoses during gastrointestinal reconstruction were performed endoscopically. Ileostomy was specified as the preventive loop ileostomy performed following colorectal anastomosis. We removed all missing data, leaving complete data for analysis.
A multidisciplinary team comprising surgeons, oncologists, and radiologists determined appropriate treatment strategies, considering patient preferences for neoadjuvant therapy. Colorectal surgery was conducted using the da Vinci Surgical System XI (Intuitive Surgical, Sunnyvale, CA) or laparoscopic system (Karl Storz SE & Co. KG, Tuttlingen, Germany). Anastomosis procedures included ileo-colic, colo-colic, and colo-rectal anastomoses. The abdominal cavity was meticulously examined, revealing the surgical area with precise tumor location. Local lymph nodes were meticulously cleared via vascularization. The target tumor and intestinal canal were dissected and resected. Following intestinal anastomosis, the specimen was extracted through either a small abdominal incision or a natural orifice. All surgeries were conducted by a consistent surgical team. The lead surgeon, with extensive experience in more than 500 laparoscopic colorectal surgeries, had overcome the learning curve for robotic colorectal surgery before embarking on the operations.
Baseline characteristics were evaluated using descriptive statistics. Measurement data with a normal distribution were expressed by x ± s, while skewed distribution is expressed as M(IQR). Group comparisons were conducted using the independent samples t-test or Mann–Whitney U test for measurement data, and x2 test or Fisher’s exact probability method for categorical data.
The research outcome was the incidence of POGD and the short-term outcomes of minimally invasive surgery. In the statistical analysis, potential influencing factors included demographic and surgical indicators. Robotic surgery was considered a potential regulatory factor. Univariable logistic regression models were employed to assess the association between POGD and patient characteristics. Variables with a P-value < 0.20 in the univariable analyses were included in the multivariable regression models to identify independent risk factors, employing backward step selection. Linear regression models were developed to assess the impact of POGD on the length of stay and hospital cost. We also employed hierarchical multiple regression to explore the potential interactions between POGD, surgery platform, and short-term outcomes. Subgroup analyses were performed to investigate whether the relationship between POGD and short-term outcomes depended on the surgical platform. Continuous variable data were discretized into categorical variables based on clinical experience and prior research [13, 14].
In the sensitivity analyses, a 1:2 propensity score matching (PSM) was employed with a caliper width of 0.25 to control biases in the baseline characteristics between the robotic and laparoscopic groups. Multivariate regression was used to determine the risk factors for POGD after PSM. Tests of interaction were performed for sex, age (< 70 years or ≥ 70 years), hypertension, diabetes, ileostomy, surgical time exceeding 240 min, intraoperative fluid administration (> 2000 ml or ≤ 2000 ml), anastomosis formation, and the number of lymph node resections (classified by quartiles and dichotomized as < 12 or ≥ 12). Adjustments were made for sex, anastomosis formation, and surgical time in the multivariable logistic regression model.
A P-value < 0.05 was considered statistically significant. Data processing and analysis were performed using R (4.4.0) and SPSS 25.0 (IBM), along with Zstats v0.90 (www.medsta.cn/software).
Results
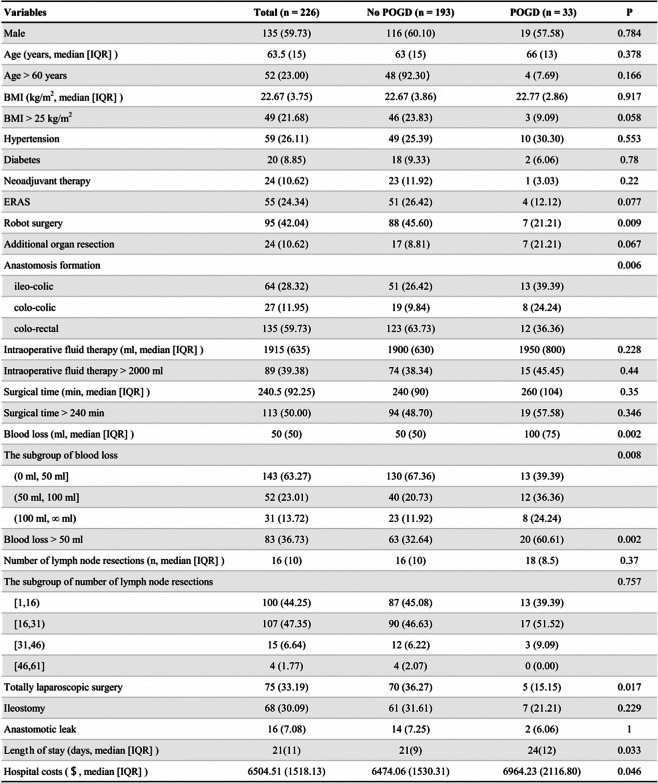
This retrospective study included 226 patients: 95 and 131 undergoing robotic and laparoscopic surgery, respectively. Among these patients, there were no cases of conversion to open surgery, multiple tumors, non-cancerous masses, or missing data. 226 patients were included in the analysis. The median operation time was 240.5 min (IQR = 92.3), and the median blood loss was 50 mL (IQR = 50). Additionally, 24.3% followed the ERAS protocol, with a median length of stay of 21 days. The patient demographic and clinical characteristics are shown in Tables 1 and 2.
Table 1.
Demographical characteristics and clinical data of the patients
A bold P value indicates statistical significance (P < 0.05). Values are represented as n(%), unless indicated otherwise
Abbreviations: BMI Body Mass Index, ERAS Enhanced Recovery After Surgery, IQR Interquartile Range
Table 2.
Short-term outcomes in 226 patients with robot group and laparoscopic group
Z Mann–Whitney test
The overall incidence of POGD was 14.6%, with 7.3% (7/95) in the robotic group versus 19.8% (26/131) in the laparoscopic group (P = 0.009). All patients with POGD experienced symptom relief following gastrointestinal decompression, eliminating the need for surgical intervention.
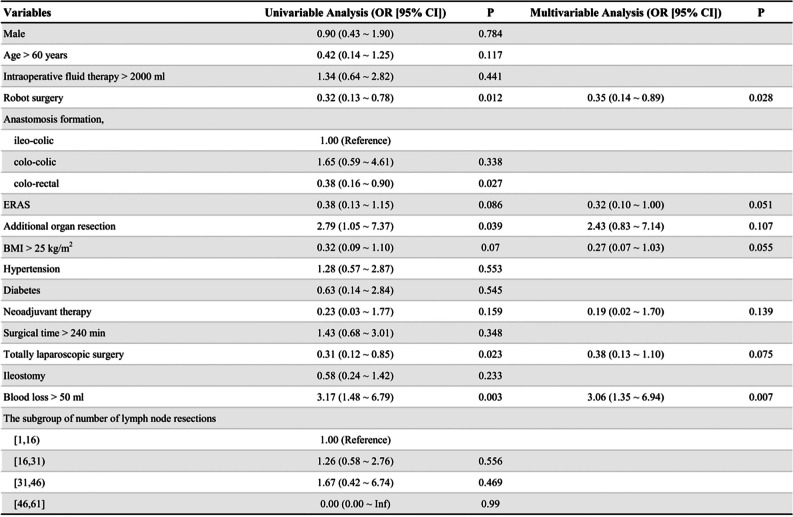
The identified potential predictors of POGD in the univariable analysis included age, surgical platform, ERAS, additional organ resection, body mass index, neoadjuvant therapy, blood loss, intracorporeal anastomosis, and surgical procedure. In the multivariable analysis, robot surgery was identified as a protective factor (OR: 0.35, 95% CI: 0.14–0.89, P = 0.028), whereas blood loss exceeding 50 ml was identified as an independent risk factor (OR: 3.06, 95% CI: 1.35–6.94, P = 0.007) (Table 3).
Table 3.
Multivariable logistic regression odds ratios for POGD
Bold numbers in univariable analysis indicates variables that were entered in multivariable anakysis (P < 0.20). Multivariable analysis after backward step selection in 226 patients. Bold numbers in multivariable analysis indicates statistical significance (P < 0.05). Values are represented as n (%), unless indicated otherwise
Abbreviations: OR Odds Ratio, CI Confidence Interval, POGD Postoperative Gastrointestinal Dysfunction, BMI Body Mas Index, ERAS Enhanced Recovery After Surgery
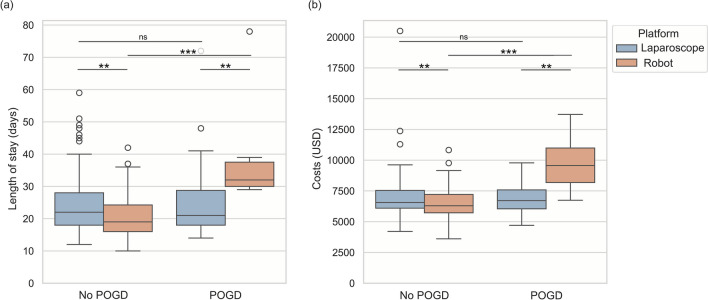
POGD was positively associated with length of stay, but not with costs. In robotic surgery, a significant difference in hospitalization time and cost was observed between patients with and without POGD, as shown in Fig. 1 (P < 0.001). This difference was not seen in laparoscopic surgery (P = 0.897 and P = 0.849, respectively). Subgroup analyses revealed that POGD was associated with an increased risk of longer length of stay in robot surgery (β = 18.15, P < 0.001), as shown in Fig. 2, but not in laparoscopic surgery (β = 1.45, P = 0.482). Similar to the length of stay, POGD was significantly related with hospital costs in robotic surgery (Fig. 3), but not in laparoscopic surgery (β = -278.59, P = 0.460).
Fig. 1.
Box Plot of lengh of stay (a) and costs (b)
Fig. 2.
Linear regression for POGD on lengh of stay (overall and subgroups). The model adjusted for potential confounders, including sex, age, surgical time > 240 min, additional organ resection, anastomos formation, blood loss > 50 ml, ileostomy and anastomotic leak
Fig. 3.
Linear regression for POGD on costs (overall and subgroups). The model adjusted for potential confounders, including neoadjuvant therapy, ERAS, robotic surgery, additional organ resection, surgical time > 240 min, anastomosis formation, ileostomy and blood loss > 50 ml
In the hierarchical multiple regression model, variables with a P-value < 0.20 in the univariable linear regression analysis set were taken as control variables, employing backward step selection. Control variables were entered in Model 1, followed by main effect variables in Model 2, and interaction terms in Model 3 (Table 4). Robot surgery had a positive moderating effect on the impact of POGD on length of stay in Table 4 (β = 11.882, P for interaction = 0.004). Similarly, the positive regulatory effect of robotic surgery was observed in the impact of POGD on hospital costs in Table 5 (β = 2957.363, P for interaction < 0.001).
Table 4.
Hierarchical multiple regression results of POGD on length of stay
Table 5.
Hierarchical multiple regression results of POGD on cost
In the sensitivity analyses, multiple regression was performed on 206 patients after PSM. In the multi model regression of 226 patients, sex, anastomosis formation, and surgical time was additionally adjusted. The risk estimates did not change materially (Table S2 and Table S4 in the Supplementary Appendix). Additionally, there were no significant interactions in any of the 10 predefined subgroups (Table S3 in the Supplementary Appendix).
Discussion
In our study, the incidence of POGD after minimally invasive colorectal surgery was 14.6%. Robotic surgery demonstrated a protective effect in regression analysis, while intraoperative bleeding exceeding 50 mL was identified as an independent risk factor. POGD was associated with a prolonged length of stay and increased costs. However, robotic surgery exacerbated the impact of POGD on extended length of stays and increased expenses.
The observed rate of POGD aligns with the reported 13%-36% range in the literature, though comparisons are difficult due to non-standardized diagnostic criteria [15–20]. Although POGD is prevalent [1], there is limited data about it in minimally invasive surgery. We used the I-FEED scoring system to diagnose POGD [11]. This system offers a more comprehensive and precise evaluation than traditional methods [21]. Notably, Yi et al. reported a higher incidence of POGD (35%) than our results, using similar criteria [22]. This discrepancy may stem from their narrow focus on laparoscopic surgery research. Previous studies have reported the positive role of robotic surgery on postoperative ileus [23, 24]. In our study, robotic surgery demonstrated advantages in reducing the rate of POGD. Given the adverse effects of POGD on patients’ quality of life and societal costs, robotic surgery emerges as a clinically favorable surgical approach [1, 25].
Blood loss exceeding 50 mL significantly increased the risk of POGD after minimally invasive colorectal surgery in our results, consistent with previous reports [26–28]. Bleeding triggers inflammation and harms gut movement via tissue damage [29, 30]. Thus, surgeons must prioritize meticulous hemostasis to prevent POGD, especially in high-risk surgeries.
Our analysis revealed that robotic surgery is a protective factor for POGD compared to laparoscopic surgery. Minimally invasive surgery is a known protective factor for POGD [15], but few previous studies have specifically examined the role of robotic surgery. Our results agreed with those of Mlambo et al.’s study of 206,967 colon surgeries. However, in contrast, our analysis adjusted clinical features and surgical procedures to minimize confounding factors. POGD is associated with multiple factors [31–34], many of which are reduced by robot surgery [35, 36]. In Hu’s research [37] robotic surgery did not impact the occurrence of POGD. Our divergent findings may stem from Hu’s exclusive focus on rectal surgery (omitting colon surgery) and the inclusion of only a small number of robotic cases. Furthermore, our study notably uncovered a higher prevalence of intracorporeal reconstruction for alimentary canals in robotic surgery than Hu’s report, which has not been addressed previously. This finding provides a potential explanation for the reduction in postoperative complications [38].
We observed a positive association between POGD and length of stay, whereas no statistically significant association was found between POGD and costs., similar to prior findings [8]. Notably, robot surgery aggravated the influence of POGD on both length of stay and hospital costs in our results. Robotic surgery has been shown to reduce the length of stay [20, 23, 30].However, this advantage may be mitigated when patients experience POGD, likely due to the prolonged surgery time in the robotic group. Prolonged surgical time presumably increases the risk of stress and complications [39–43], leading to longer length of stays and higher costs. The omission of these complications and stress in our research may explain the lack of short-term benefits seen with robotic surgery. Meanwhile, the additional equipment required for robotic surgery intensifies the financial burden associated with hospital costs [41], whereas laparoscopic surgery does not. Therefore, it is vitally important to prevent POGD during robotic surgery.
This study has several limitations due to its retrospective design, potential selection bias, post-hoc analysis, and single-center setting. Additionally, the small sample size limits the generalizability of the findings. Other confounding factors, such as prior constipation history, opioid medication use, history of COPD, inflammatory bowel disease, and other postoperative complications, were not identified. Incomplete implementation and personalized adjustments of ERAS protocols based on individual patient conditions may affect their effectiveness. Cases that underwent only partial ERAS procedures or had incomplete records of ERAS implementation were not classified as ERAS. Blood loss quantification was based solely on surgical records; there is therefore a need for more precise measurement methods in future research. The fact that this study was conducted in a developing country is crucial, in that the patients chose robotic surgery voluntarily, which may have been influenced by economic and social factors. Consequently, while this study offers valuable insights into the advantages of robotic surgery, its limitations call for further high-quality prospective research to validate the potential benefits and applications of robotic surgery.
In summary, POGD remains prevalent in minimally invasive surgeries, with robotic surgery exhibiting a lower incidence than laparoscopic surgery. Robotic surgery reduces the risk of POGD, while intraoperative bleeding exceeding 50 mL increases it. Robotic surgery leads to extended length of stays and increased costs associated with POGD. Preventive strategies, including meticulous hemostasis techniques and robotic surgery, should be prioritized by healthcare professionals to mitigate POGD risk, improve short-term outcomes, and preserve healthcare resources.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Author contributions
GZ, SP, and SY contributed equally to this article. Conceptualization: GZ, SP, SY and DW; methodology: JW and JR; writing—original draft: GZ, SP and SY; writing—review and editing: GZ, SP, SY, JW, JR and DW; visualization: SY and JW; supervision: JR and DW; funding acquisition: JR and DW.
Funding
This study was supported by the grants from the Guangxi Zhuang Autonomous Region Health Commission (Z-B20231432, Z20210069), the grants from the Guangxi Zhuang Autonomous Region Traditional Chinese Medicine Administration (GXZYB20220430), and the grant from the Liuzhou Science and Technology Plan (2022SB019).
Data Availability
No datasets were generated or analysed during the current study.
Declarations
Ethics statement
Since all participants receiving minimally invasive colorectal surgery were informed that perioperative clinical data might be retrospectively analyzed and published, and the data were collected as part of standard surgical care. This study protocol was approved by the Ethical Committee of the Fourth Affiliated Hospital of Guangxi Medical University (no. KY2022260).
Competing interest
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Guiqi Zhang, Shiquan Pan, and Shengfu Yang contributed equally to this article.
References
- 1.Pozios I, Seeliger H, Lauscher JC et al (2021) Risk factors for upper and lower type prolonged postoperative ileus following surgery for Crohn’s disease. Int J Colorectal Dis 36(10):2165–2175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Millan M, Biondo S, Fraccalvieri D et al (2012) Risk factors for prolonged postoperative ileus after colorectal cancer surgery. World J Surg 36(1):179–185 [DOI] [PubMed] [Google Scholar]
- 3.Vather R, Josephson R, Jaung R et al (2015) Development of a risk stratification system for the occurrence of prolonged postoperative ileus after colorectal surgery: a prospective risk factor analysis. Surgery 157(4):764–773 [DOI] [PubMed] [Google Scholar]
- 4.Chapuis PH, Bokey L, Keshava A et al (2013) Risk factors for prolonged ileus after resection of colorectal cancer: an observational study of 2400 consecutive patients. Ann Surg 257(5):909–915 [DOI] [PubMed] [Google Scholar]
- 5.Wolthuis AM, Bislenghi G, Fieuws S et al (2016) Incidence of prolonged postoperative ileus after colorectal surgery: a systematic review and meta-analysis. Colorectal Dis 18(1):O1-9 [DOI] [PubMed] [Google Scholar]
- 6.Quiroga-Centeno AC, Jerez-Torra KA, Martin-Mojica PA et al (2020) Risk Factors for Prolonged Postoperative Ileus in Colorectal Surgery: A Systematic Review and Meta-analysis. World J Surg 44(5):1612–1626 [DOI] [PubMed] [Google Scholar]
- 7.Mao H, Milne TGE, O’Grady G et al (2019) Prolonged Postoperative Ileus Significantly Increases the Cost of Inpatient Stay for Patients Undergoing Elective Colorectal Surgery: Results of a Multivariate Analysis of Prospective Data at a Single Institution. Dis Colon Rectum 62(5):631–637 [DOI] [PubMed] [Google Scholar]
- 8.Traeger L, Koullouros M, Bedrikovetski S et al (2022) Cost of postoperative ileus following colorectal surgery: A cost analysis in the Australian public hospital setting. Colorectal Dis 24(11):1416–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin Z, Yang C, Wang Y et al (2022) Comparison of prolonged postoperative ileus between laparoscopic right and left colectomy under enhanced recovery after surgery: a propensity score matching analysis. World J Surg Oncol 20(1):68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cuk P, Kjær MD, Mogensen CB et al (2022) Short-term outcomes in robot-assisted compared to laparoscopic colon cancer resections: a systematic review and meta-analysis. Surg Endosc 36(1):32–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hedrick TL, McEvoy MD, Mythen MMG et al (2018) American Society for Enhanced Recovery and Perioperative Quality Initiative Joint Consensus Statement on Postoperative Gastrointestinal Dysfunction Within an Enhanced Recovery Pathway for Elective Colorectal Surgery. Anesth Analg 126(6):1896–1907 [DOI] [PubMed] [Google Scholar]
- 12.Gustafsson UO, Scott MJ, Hubner M et al (2019) Guidelines for Perioperative Care in Elective Colorectal Surgery: Enhanced Recovery After Surgery (ERAS®) Society Recommendations: 2018. World J Surg 43(3):659–695 [DOI] [PubMed] [Google Scholar]
- 13.WHO, Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Consultation. WHO Technical ReportSeries Number 854. Geneva: World Health Organization, 1995. [PubMed]
- 14.Huang L, Yin Y, Liao Y et al (2022) Risk factors for postoperative urinary retention in patients undergoing colorectal surgery: a systematic review and meta-analysis. Int J Colorectal Dis 37(12):2409–2420 [DOI] [PubMed] [Google Scholar]
- 15.Sapci I, Hameed I, Ceylan A et al (2020) Predictors of ileus following colorectal resections. Am J Surg 219(3):527–529 [DOI] [PubMed] [Google Scholar]
- 16.Rybakov EG, Shelygin YA, Khomyakov EA et al (2018) Risk factors for postoperative ileus after colorectal cancer surgery[J]. Colorectal Dis: Off J Assoc Coloproctol Great Britain Ireland 20(3):189–194 [DOI] [PubMed] [Google Scholar]
- 17.Koch KE, Hahn A, Hart A et al (2021) Male sex, ostomy, infection, and intravenous fluids are associated with increased risk of postoperative ileus in elective colorectal surgery. Surgery 170(5):1325–1330 [DOI] [PubMed] [Google Scholar]
- 18.Watkins EL, Schellack N, Abraham V et al (2021) Men and Those With a History of Smoking Are Associated With the Development of Postoperative Ileus Following Elective Colorectal Cancer Resection at a Private Academic Hospital in Johannesburg, South Africa: A Retrospective Cohort Study. Front Surg 15(8):667124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kronberg U, Kiran RP, Soliman MS et al (2011) A characterization of factors determining postoperative ileus after laparoscopic colectomy enables the generation of a novel predictive score. Ann Surg 253(1):78–81 [DOI] [PubMed] [Google Scholar]
- 20.Alsharqawi N, Alhashemi M, Kaneva P et al (2020) Validity of the I-FEED score for postoperative gastrointestinal function in patients undergoing colorectal surgery. Surg Endosc 34(5):2219–2226 [DOI] [PubMed] [Google Scholar]
- 21.Yi M, Wu Y, Li M et al (2023) Effect of remote ischemic preconditioning on postoperative gastrointestinal function in patients undergoing laparoscopic colorectal cancer resection. Int J Colorectal Dis 38(1):68 [DOI] [PubMed] [Google Scholar]
- 22.Zhu XL, Yan PJ, Yao L et al (2019) Comparison of short-term outcomes between robotic-assisted and laparoscopic surgery in colorectal cancer. Surg Innov 26(1):57–65 [DOI] [PubMed] [Google Scholar]
- 23.G Palomba, VP Dinuzzi, M Capuano et al (2022) Robotic versus laparoscopic colorectal surgery in elderly patients in terms of recovery time:a monocentric experience. J Robot Surg 16(4):981–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Butnari V, Sultana M, Mansuri A et al (2024) Comparison of early surgical outcomes of robotic and laparoscopic colorectal cancer resection reported by a busy district general hospital in England. Sci Rep 14(1):9227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peters EG, Pattamatta M, Smeets BJJ et al (2020) The clinical and economical impact of postoperative ileus in patients undergoing colorectal surgery. Neurogastroenterol Motil 32(8):e13862 [DOI] [PubMed] [Google Scholar]
- 26.Alhashemi M, Fiore JF Jr, Safa N et al (2019) Incidence and predictors of prolonged postoperative ileus after colorectal surgery in the context of an enhanced recovery pathway. Surg Endosc 33(7):2313–2322 [DOI] [PubMed] [Google Scholar]
- 27.Courtot L, Le Roy B, Memeo R et al (2018) Risk factors for postoperative ileus following elective laparoscopic right colectomy: a retrospective multicentric study. Int J Colorectal Dis 33(10):1373–1382 [DOI] [PubMed] [Google Scholar]
- 28.He Y, Wang J, Bian H et al (2017) BMI as a Predictor for Perioperative Outcome of Laparoscopic Colorectal Surgery: a Pooled Analysis of Comparative Studies. Dis Colon Rectum 60(4):433–445 [DOI] [PubMed] [Google Scholar]
- 29.Wehner Sven, Vilz Tim O, Stoffels Burkhard et al (2012) Immune mediators of postoperative ileus. Langenbeck’s Archives Surg 397(4):591–601 [DOI] [PubMed] [Google Scholar]
- 30.van Bree SHW, Cailotto C, Di Giovangiulio M et al (2013) Systemic inflammation with enhanced brain activation contributes to more severe delay in postoperative ileus[J]. Neurogastroenterol Motil 25(8):e540–e549 [DOI] [PubMed] [Google Scholar]
- 31.Millan M, Biondo S, Fraccalvieri D, Frago R, Golda T, Kreisler E (2012) Risk factors for prolonged postoperative ileus after colorectal cancer surgery. World J Surg 36:179–185 [DOI] [PubMed] [Google Scholar]
- 32.Vather R, Bissett IP (2013) Risk factors for the development of prolonged post-operative ileus following elective colorectal surgery. Int J Colorectal Dis 28:1385–1391 [DOI] [PubMed] [Google Scholar]
- 33.Artinyan A, Nunoo-Mensah JW, Balasubramaniam S et al (2008) Prolonged postoperative ileus-definition, risk factors, and predictors after surgery. World J Surg 32:1495–1500 [DOI] [PubMed] [Google Scholar]
- 34.Boeckxstaens GE, de Jonge WJ (2009) Neuroimmune mechanisms in postoperative ileus. Gut 58(9):1300–1311 [DOI] [PubMed] [Google Scholar]
- 35.Ravindra C, Igweonu-Nwakile EO, Ali S et al (2022) Comparison of Non-Oncological Postoperative Outcomes Following Robotic and Laparoscopic Colorectal Resection for Colorectal Malignancy: A Systematic Review and Meta-Analysis. Cureus 14(7):e27015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chaouch Mohamed Ali, MDa; Daghmouri, et al (2023) How to prevent postoperative ileus in colorectal surgery? a systematic review. Annals of Medicine & Surgery 85(9):4501–4508 [DOI] [PMC free article] [PubMed]
- 37.Hu Q, Oki E, Fujimoto Y et al (2022) Influence of Robotic Rectal Resection Versus Laparoscopic Rectal Resection on Postoperative Ileus: A Single-center Experience. Surg Laparosc Endosc Percutan Tech 32(4):425–430 [DOI] [PubMed] [Google Scholar]
- 38.Zhang T, Sun Y, Mao W (2023) Meta-analysis of randomized controlled trials comparing intracorporeal versus extracorporeal anastomosis in minimally invasive right hemicolectomy: upgrading the level of evidence. Int J Colorectal Dis 38(1):147 [DOI] [PubMed] [Google Scholar]
- 39.Feng Q, Tang W, Zhang Z et al (2022) Robotic versus laparoscopic abdominoperineal resections for low rectal cancer: A single-center randomized controlled trial. J Surg Oncol 126(8):1481–1493 [DOI] [PubMed] [Google Scholar]
- 40.Peyton PJ, Wu CY et al (2014) Nitrous oxide-related postoperative nausea and vomiting depends on duration of exposure. Anesthesiology 120(5):1137–1145 [DOI] [PubMed] [Google Scholar]
- 41.Mukaida H, Matsushita S, Minami Y et al (2022) Risk factors for postoperative delirium on oxygen delivery-guided perfusion. J Cardiothorac Surg 17(1):193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guidolin K, Spence RT, Azin A et al (2022) The effect of operative duration on the outcome of colon cancer procedures. Surg Endosc 36(7):5076–5083 [DOI] [PubMed] [Google Scholar]
- 43.Kurmann A, Vorburger SA, Candinas D et al (2011) Operation time and body mass index are significant risk factors for surgical site infection in laparoscopic sigmoid resection: a multicenter study. Surg Endosc 25(11):3531–3534 [DOI] [PubMed] [Google Scholar]
- 44.Merola G, Sciuto A, Pirozzi F et al (2020) Is robotic right colectomy economically sustainable? a multicentre retrospective comparative study and cost analysis. Surg Endosc 34(9):4041–4047 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.