ABSTRACT
Introduction
Transplant vasculopathy resembles atherosclerotic plaque formation and is a major contributor to late graft failure in kidney transplant recipients (KTR). Remnant lipoproteins and associated triglycerides are causal risk factors for atherosclerotic plaques and have been implicated in late kidney graft failure. However, whether remnants derived from liver (containing apolipoprotein [apo] B100) or intestine (containing apoB48) are clinically more important is unclear. The current study investigated the association between baseline fasting apoB48 levels and late kidney graft failure.
Methods
481 KTR with a functioning graft for at least 1 year were included in this retrospective, observational longitudinal single center cohort study. The primary endpoint was death-censored late graft failure, defined as need for initiation of dialysis or re-transplantation. ApoB48 was measured by enzyme-linked immunosorbent assay.
Results
During a median follow-up of 9.5 years, 61 KTR developed graft failure (12.7%). At baseline, KTR with higher apoB48 levels had lower eGFR (P < .001), lower high-density lipoprotein (HDL) cholesterol (P < .001), increased triglycerides (P < .001) and used cyclosporine more frequently (P = .003). Cox regression showed that higher baseline apoB48 was associated with higher risk of late graft failure [hazard ratio (95% confidence interval), 1.59 (1.22, 2.07), P < .001], independent of stepwise adjustment for potential confounders, including age and sex, immunosuppression type and proteinuria, triglycerides, and waist circumference (fully adjusted HR, 1.78 (1.29, 2.47), P < .001].
Conclusion
ApoB48 is strongly associated with late graft failure, independent of potential confounders. Since apoB48-containing lipoproteins originate from the intestine, this study provides a rationale for considering pharmacological interventions targeting lipid absorption to improve graft outcome.
Keywords: Apolipoprotein B-48, dyslipidemia, kidney transplantation, late graft failure, prospective longitudinal cohort study
KEY LEARNING POINTS.
What was known:
Transplant vasculopathy resembles atherosclerotic plaque formation in kidney transplant recipients (KTR).
Transplant vasculopathy is a major contributor to late graft failure.
Remnant lipoproteins originate either from intestine or liver and are atherogenic.
This study adds:
As judged by circulating levels of apolipoprotein B-48 (apoB48) KTR have increased fasting intestine-derived remnants.
Higher apoB48 concentrations are associated with a higher risk of death-censored late kidney graft failure.
Potential impact:
ApoB48 is a novel biomarker for late kidney graft failure.
These results provide a rationale for considering pharmacological interventions aiming at reducing remnant lipoprotein levels in clinical practice.
INTRODUCTION
The incidence and prevalence of chronic kidney disease (CKD) are globally increasing [1]. CKD is a progressive condition predisposing a substantial fraction of affected patients to eventually develop kidney failure, requiring kidney replacement therapy. Kidney transplantation is the preferred treatment of patients with kidney failure, improving both life expectancy and quality of life [2]. Consequently, the population of kidney transplant recipients (KTR) is continuously expanding [1]. Owing to improved immediate post-surgical care and better immunosuppressive medication regimens, the clinical importance of acute allograft rejection is decreasing, shifting the focus to preventing long-term adverse graft and patient outcomes [3]. Late graft failure is such an adverse outcome, associated with significant morbidity and mortality [4]. KTR with failed kidney allografts require kidney replacement therapy, typically in the form of dialysis or re-transplantation [4]. The management of patients with a failed kidney allograft is challenging and can be further complicated by a state of chronic inflammation referred to as graft intolerance syndrome when the graft is left in situ while stopping immunosuppressive medication [4]. Notably, KTR typically perceive late graft failure followed by dialysis a worse clinical outcome than death [5]. Yet, implementing personalized medicine strategies to prevent late graft failure particularly by identifying biomarkers amenable to therapy represents an unmet clinical need. This is partly driven by the incomplete understanding of the pathogenesis of late graft failure. Allograft vasculopathy, a condition that resembles atherosclerotic plaque formation, appears to be a major contributor [6]. This is illustrated by the notion that the Framingham Risk Score is a strong predictor of late graft failure [7]. Next to low-density lipoprotein cholesterol (LDL-C), triglycerides and remnant cholesterol are emerging factors contributing to atherosclerotic plaque formation [8]. Remnants are incompletely lipolyzed, thus triglyceride-rich, lipoproteins derived from either apoB100-containing very low-density lipoproteins (VLDL) secreted by the liver or apoB48-containing chylomicrons derived from the intestine [9]. Previous work suggested that dyslipidemia in the form of elevated triglycerides and low high-density lipoprotein cholesterol (HDL-C) contributes significantly to late graft failure [10]. However, the source of the remnant particles carrying these triglycerides is unclear. Such knowledge has potential therapeutic implications since it could inform the choice of an efficient pharmacological intervention strategy [11, 12, 13]. Therefore, the current study examined the association between baseline fasting apoB48 levels and late graft failure in KTR.
MATERIALS AND METHODS
Study design
Patients with a functioning allograft for at least one year were recruited to participate in the current retrospective, observational single center cohort study between August 2001 and July 2003 in the University Medical Center Groningen [14]. Of 847 eligible patients, 606 KTR provided written informed consent. Clinical data from donors and KTR were obtained from the Groningen Renal Transplant Database. Patients diagnosed with systemic illnesses such as congestive heart failure, endocrine disorders other than diabetes, generalized infections, or cancer (not including cured skin cancer) were excluded. Furthermore, patients with missing plasma samples (n = 120) or with missing follow-up (n = 6) were excluded, leaving 481 eligible KTR for the final analysis (Supplementary Figure 1). Plasma samples from 19 healthy volunteers were from a previously published study [15] and assayed along with the KTR samples for apoB48 levels. The study was carried out in accordance with the Declaration of Helsinki and has been approved by the Medical Ethical Institutional Review Board of the University Medical Center Groningen (METc 01/039).
Study population
From all KTR included, 431 (89.6%) were transplanted once, 44 (9.1%) were transplanted twice, three (0.6%) were transplanted three times, and three (0.6%) were transplanted four times. Prior to undergoing transplantation, CKD was caused by: primary glomerulonephritis in 130 (27.0%) KTR, glomerulonephritis due to vasculitis or autoimmune disease in 32 (6.7%) KTR, tubulointerstitial nephritis or pyelonephritis in 75 (15.6%) KTR, polycystic kidney disease in 83 (17.3%) KTR, dysplasia or hypoplasia in 18 (3.7%) KTR, renovascular disease in 31 (6.4%) KTR, diabetes in 16 (3.3%) KTR, and genetic disease or unknown cause in 96 (20.0%) KTR.
Standard laboratory measurements
Blood samples were drawn after an overnight fast. Fasting glucose was measured using capillary glucose testing at all routine follow-up visits (glucose-oxidase method, YSI 2300 Stat Plus; YSI Incorporated, Yellow Springs, OH, USA). The Chronic Kidney Disease Epidemiology Collaboration equations were used to calculate estimated glomerular filtration rate (eGFR) [16]. Furthermore, using routine clinical chemistry methods, total cholesterol, high-density lipoprotein (HDL)-C, triglycerides, and plasma insulin were measured. HbA1c was measured by high-performance liquid chromatography (VARIANT Hemoglobin Testing System; Bio-Rad Laboratories, Hercules, CA, USA). HOMA of insulin resistance (HOMA-IR) was calculated by the following formula: HOMA-IR = glucose (mmol/l) × insulin (μU/ml)/22.5. The Friedewald equation was used to calculate LDL-C. Apolipoprotein A-I (apoA-I) and total apoB were measured by immunoturbidimetry (COBAS INTEGRA System, Roche Diagnostics, Mannheim, Germany). A specific enzyme-linked immunosorbent assay (ELISA) was used to determine plasma hs-CRP (C-reactive protein). Urinary protein excretion was measured by the Biuret reaction (MEGA AU 510; Merck).
Measurement of apo B-48
Fasting apoB48 concentrations were determined using ELISA in EDTA plasma samples centrifuged immediately at 4°C and stored at −80°C after collection following the protocol supplied by the manufacturer (FUJIFILM Wako Shibayagi Corporation, Osaka, Japan).
Baseline characteristics
Blood pressure was measured three times after ≥6 minutes of rest in a supine position using an automated device (OMRON M4; OMRON Healthcare Europe B.V., Hoofddorp, the Netherlands). The average of the measurements was calculated. Waist circumference was measured on bare skin between the iliac crest and the tenth rib. BMI was calculated as weight (kilograms) divided by height in meters squared.
Outcome definitions
Graft failure was defined as the clinical need to initiate kidney replacement therapy such as dialysis or re-transplantation. Patients were censored at death. Cardiovascular morbidity and time to cardiovascular events was recorded during the study period. Data regarding cardiovascular events were obtained from the medical records of patients and were defined as the first occurrence of a cardiovascular event or cardiovascular death. Ischemic events were defined as myocardial infarction, cerebral infarction, transient ischemic attacks, coronary artery bypass grafting, and percutaneous transluminal coronary angioplasty. Mortality was recorded until the end of the study, which was 1 April 2012.
Statistical analysis
P values <.05 were considered statistically significant. R studio (RStudio, PBC, Boston, MA, USA) and R (version 4.1.2) were used to conduct all statistical analyses. To increase reproducibility and transparency, the paper has been written in R markdown and all code used for statistical analysis has been made publicly available from github.com/tamas875/research-source-codes. Normal distribution was checked for all variables utilizing Q-Q plots. Median [interquartile range] and mean [standard deviation] were used to present data with skewed distributions and normal distributions, respectively. Categorical variables are presented as absolute numbers. To evaluate the prospective association between apoB48 and death-censored late graft failure, Cox proportional hazards regression analysis was done. First, to adjust for potential confounders, several models were constructed in a stepwise manner. Hazard ratios are presented per doubling of fasting apoB48. Model 1 represents the crude analysis, model 2 was adjusted for age and sex, model 3 represents model 2 + proteinuria and immunosupressive medication use, model 4 represents model 3 + triglyceride concentrations, model 5 represents model 4 + waist circumference, model 6 represents model 5 + CRP, pre-transplant kidney disease diagnosis, statin use and prednisolone dose, and model 7 represents model 6 + daily fruit and vegetable intake (please note that dietary information was only available in 66% of the study participants). Schoenfeld residuals showed that the proportional hazards assumption was not violated. Subsequently, interaction tests were performed. The P value for interaction effect was estimated by a likelihood ratio test. The continuous association between baseline apoB48 concentrations and adjusted (age and sex) hazard ratios for death-censored graft failure was visualized by using cubic splines with four knots. The ideal number of knots was determined by obtaining the Akaike information criterion for models with 3–7 knots and the model with the best fit was chosen. A ratio was calculated between apoB48 and triglycerides followed by Cox proportional hazards regression analysis with a fully adjusted model. Next, the association between the apoB48/triglycerides ratio and graft failure was visualized using cubic splines. Last, mediation analysis was carried out to investigate whether kidney function as measured by eGFR is a (full) mediator between apoB48 and death-censored graft failure. To investigate the prospective association between apoB48 and cardiovascular events crude Cox proportional hazards regression was performed. Last, binominal logistic regression was conducted to quantify the association between apoB48 and overall mortality.
RESULTS
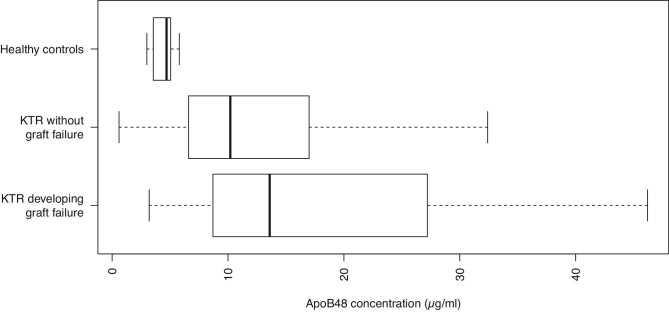
In this prospective longitudinal cohort study 481 stable KTR were included. Median [IQR] apoB48 levels were 10.5 [6.9 to 18.0] µg/ml. This was substantially higher than the median [IQR] apoB48 levels of 19 healthy volunteers [4.7 (3.5 to 5.0) µg/ml], a range very consistent with the normal values provided as orientation by Fuji, the manufacturer of the ELISA kit (Figure 1). KTR who developed graft failure had increased baseline apoB48 concentrations compared to KTR who did not [13.6 (8.7 to 27.2) µg/ml vs. 10.2 (6.6 to 16.9) µg/ml].
Figure 1:
Fasting plasma apoB48 levels in healthy individuals, KTR who developed death-censored late graft failure and KTR who did not, as indicated.
Baseline demographic characteristics
Baseline characteristics analysis (Table 1) revealed that the percentage of female participants was lower with increasing tertiles of apoB48 indicating that males had significantly higher apoB48 levels compared to females (P < .001). Fasting apoB48 was inversely associated with eGFR (P < .001), apoA-I (P = .001), and HDL cholesterol (P < .001) while positive associations were observed with waist circumference (P = .001), HbA1c (P = .007), triglycerides (P < .001), total cholesterol (P < .001), and apoB (P < .001). Of note, BMI (P = .120), glucose (P = .197) or HOMA-IR (P = .783) were not associated with apoB48. Interestingly, both calcineurin inhibitor (P = .003) and proliferation inhibitor (P = .016) use correlated significantly with apoB48 levels, while statin use was not (P = .098); 479 (99.6%) of participants used prednisolone and seven KTR (1.5%) used mTOR inhibitors at baseline, whereas five (1.0%) KTR received a combination therapy of mTOR inhibitors and cyclosporine. Information related to induction therapy is shown in Supplementary Table 1.
Table 1:
Baseline characteristics per tertiles of apoB48.
| Low tertile (N = 163) 0.6–7.9 µg/ml | Medium tertile (N = 159) 8.0–15.0 µg/ml | High tertile (N = 159) 15.1–46.2 µg/ml | P value | |
|---|---|---|---|---|
| Age (years) | 52.8 [43.3–60.7] | 55.1 [42.5–62.3] | 52.2 [44.4–58.1] | .332 |
| Sex, N (%) (Female) | 102 (62.6) | 70 (44.0) | 46 (28.9) | <.001 |
| Time since transplantation (years) | 4.6 [1.3–11.0] | 4.6 [1.7–9.7] | 6.5 [2.5–10.9] | .155 |
| Creatinine (µmol/l) | 120.0 [103.0–142.0] | 136.0 [111.0–164.0] | 152.0 [128.0–183.5] | <.001 |
| Protein excretion (g/24 h) | 0.2 [0.0–0.4] | 0.2 [0.0–0.5] | 0.3 [0.1–0.6] | .002 |
| eGFR (ml/min/1.73 m2) | 51.8 [42.8–61.1] | 48.0 [36.1–56.7] | 41.0 [32.1–53.9] | <.001 |
| Time on dialysis (months) | 30.0 [14.0–48.0] | 29.0 [16.0–50.0] | 24.0 [11.5–46.5] | .294 |
| Total number of HLA mismatches | 2.0 [0.0–2.5] | 2.0 [1.0–3.0] | 2.0 [1.0–2.5] | .600 |
| BMI (kg/m2) | 24.9 [22.5–27.8] | 25.5 [23.6–29.0] | 26.0 [23.7–28.0] | .120 |
| Waist circumference (cm) | 93.7 ± 13.8 | 97.6 ± 13.8 | 99.1 ± 13.1 | .001 |
| Smoking (yes) | 74 (46.0) | 49 (31.0) | 70 (44.0) | .013 |
| Dietary fruit intake (cups per day) | 1.0 [1.0 to 2.0] | 1.0 [1.0 to 2.0] | 1.0 [1.0 to 2.0] | .796 |
| Dietary vegetable intake (cups per day) | 3.0 [2.0 to 3.0] | 2.0 [2.0 to 3.0] | 2.0 [2.0 to 3.0] | .821 |
| Systolic blood pressure (mmHg) | 148.0 [131.0–165.0] | 151.0 [139.5–164.5] | 151.0 [136.0–167.0] | .183 |
| Diastolic blood pressure (mmHg) | 88.2 ± 10.4 | 90.3 ± 9.7 | 90.4 ± 9.7 | .081 |
| hs-CRP (mg/dl) | 1.9 [0.8–4.3] | 2.1 [1.1–4.8] | 1.9 [0.7–4.1] | .326 |
| Diabetes N (%) (yes) | 51 (31.3) | 42 (26.4) | 54 (34.0) | .334 |
| Myocardial infarction history N (%) | 13 (8.1) | 12 (7.6) | 16 (10.1) | .695 |
| Cerebrovascular accident or transient ischemic attack history N (%) | 13 (8.1) | 4 (2.5) | 8 (5.1) | .084 |
| Insulin (µmol/l) | 11.2 [8.3–16.6] | 11.0 [7.8–14.8] | 10.6 [7.7–14.7] | .559 |
| HbA1c (%) | 6.1 [5.7–6.7] | 6.4 [5.8–6.9] | 6.4 [6.0–7.2] | .007 |
| HOMA-IR | 2.3 [1.6–3.5] | 2.2 [1.5–3.3] | 2.2 [1.6–3.1] | .783 |
| Statin use N (%) (yes) | 75 (46.0) | 76 (47.8) | 91 (57.2) | .098 |
| Glucose (mmol/l) | 4.4 [4.0–5.0] | 4.5 [4.1–5.0] | 4.6 [4.1–5.0] | .197 |
| Total cholesterol (mmol/l) | 5.4 ± 0.9 | 5.7 ± 1.0 | 5.9 ± 1.2 | <.001 |
| HDL cholesterol (mmol/l) | 1.2 [1.0–1.4] | 1.1 [0.9–1.3] | 1.0 [0.8–1.1] | <.001 |
| Triglycerides (mmol/l) | 1.4 [1.1–1.9] | 1.9 [1.4–2.4] | 2.5 [1.8–3.2] | <.001 |
| Apo A-I (g/dl) | 1.6 [1.4–1.8] | 1.5 [1.3–1.7] | 1.5 [1.3–1.7] | .001 |
| Apo B (g/dl) | 1.0 ± 0.2 | 1.1 ± 0.2 | 1.1 ± 0.2 | <.001 |
| Prednisolone dose (mg/day) | 10.0 [8.1–10.0] | 10.0 [7.5–10.0] | 10.0 [7.5–10.0] | .779 |
| Calcineurine inhibitor use N (%) | .003 | |||
| None | 46 (28.2) | 32 (20.1) | 27 (17.0) | |
| Cyclosporine | 91 (55.8) | 105 (66.0) | 121 (76.1) | |
| Tacrolimus | 26 (16.0) | 22 (13.8) | 11 (6.9) | |
| Proliferation inhibitor use N (%) | .016 | |||
| No | 28 (17.2) | 45 (28.3) | 52 (32.7) | |
| Azathioprine | 64 (39.3) | 47 (29.6) | 52 (32.7) | |
| Mycophenolic acid | 71 (43.6) | 67 (42.1) | 55 (34.6) |
Normally distributed variables are given as mean ± standard deviation, skewed variables as median [interquartile range] and categorical variables as N (%). Significance was tested using one-way analysis of variance for normally distributed variables, Kruskall–Wallis test for skewed variables and χ2 test for categorical variables.
eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin; HOMA-IR, HOMA of insulin resistance; Apo A, apolipoprotein A-I; ApoB, apolipoprotein B.
Additional baseline characteristics analysis per statin use revealed that KTR who were on statins had significantly higher circulating apoB48 levels, possibly due to prescription bias [9.7 (6.0 to 16.4) µg/ml in non-statin users vs. 11.6 (7.3 to 18.7) µg/ml in all statin users combined, P = .017, Supplementary Table 2].
ApoB48 and incident death-censored graft failure
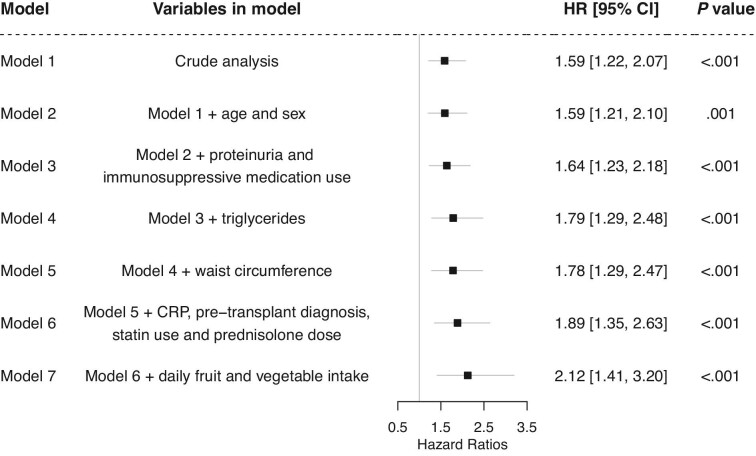
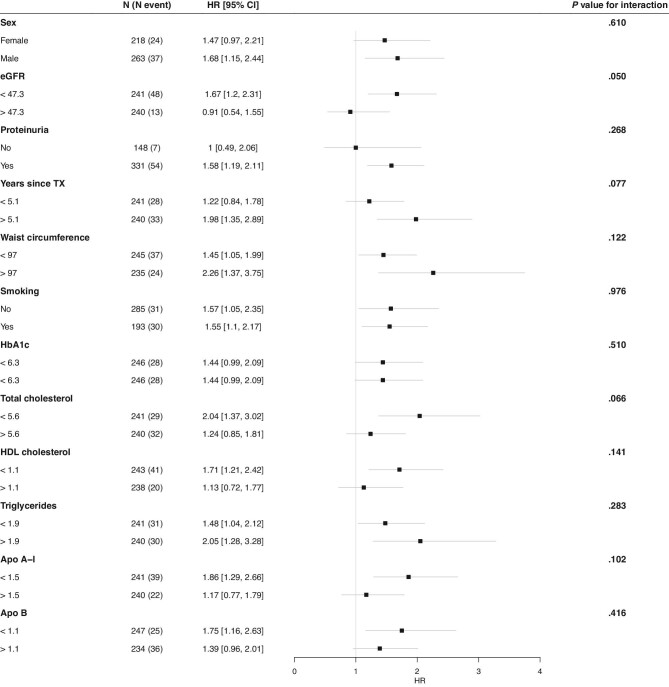
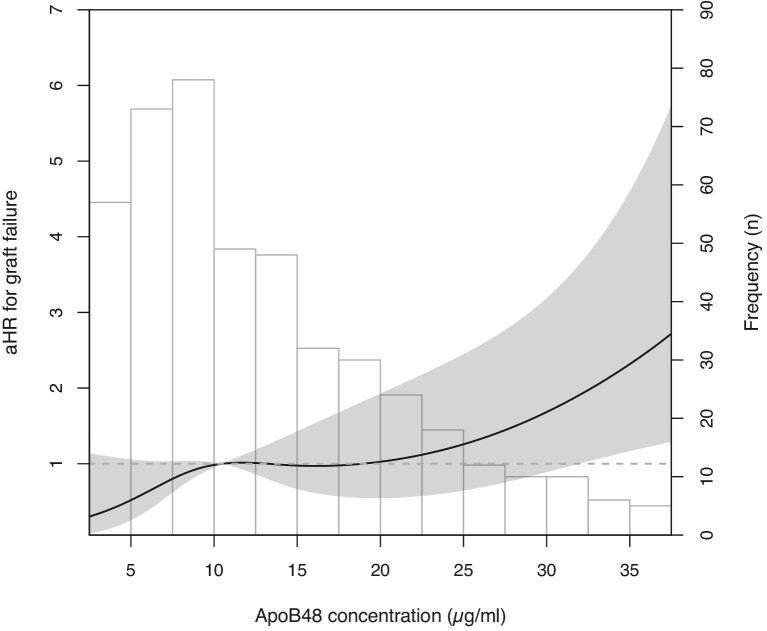
During the median [IQR] follow-up of 9.5 [6.5–10.2], years, 61 (12.7%) KTR developed death-censored graft failure. The longitudinal association between apoB48 concentrations and late kidney allograft failure was assessed using multivariate Cox proportional hazard analyses. First, several models were constructed in a stepwise manner to adjust for potential confounders (Figure 2). Crude analysis showed that a higher plasma apoB48 was significantly associated with an increased risk of death-censored graft failure [model 1, hazard ratio (HR) 1.59 (1.22, 2.07), P < .001]. This association persisted on adjusting for age and sex [model 2, HR 1.59 (1.21, 2.10), P = .001], proteinuria and immunosuppressive medication use [model 3, HR 1.64 (1.23, 2.18), P < .001], triglycerides [model 4; 1.79 (1.29, 2.48), P < .001], waist circumference [model 5, HR 1.59 (1.22, 2.07), P < .001], CRP, pre-transplant kidney disease diagnosis, statin use, prednisolone dose [model 6, HR 1.89 (1.35, 2.63), P < .001], and daily fruit and vegetable intake [model 7, HR 2.12 (1.41, 3.20), P < .001]. To visualize the prospective association between the apoB48/triglycerides ratio and late graft failure, splines analysis was done (Supplementary Figure 2). The ratio was prospectively associated with late graft failure independent of potential confounders [fully adjusted model, HR, 2.0 (1.35, 2.95), P < 0.001]. Next, interaction tests were performed (Figure 3). There were no significant interactions for the association between apoB48 and late graft failure by sex (P = .610), eGFR (P = .050), proteinuria (P = .268), years since transplantation (P = .077), waist circumference (P = .122), smoking (P = .976), HbA1c (P = .510), total cholesterol (P = .066), HDL cholesterol (P = .141), triglycerides (P = .283), apoA-I (P = .102), or apoB (P = .416). To visualize the relation between continuous apoB48 and incident late graft failure, cubic splines were plotted revealing that adjusted hazard ratios increase with increasing apoB48 concentrations (Figure 4). Analysis with splines provides insight into how increasing apoB48 levels impact the hazard ratio of graft failure, possibly uncovering non-linear associations. Mediation analysis revealed that kidney function measured by eGFR was a full mediator between apoB48 and death-censored graft failure (Figure 5). Last, total apoB plasma levels were not significantly associated with incident late graft failure [crude model, HR, 2.49 (0.84, 7.34), P = .100].
Figure 2:
ApoB48 is prospectively associated with incident death-censored late graft failure independent of potential confounders, Model 1: crude analysis, Model 2: adjusted for age and sex, Model 3: model 2 + proteinuria and calcineurin and proliferation inhibitor use, Model 4: model 3 + triglycerides, Model 5: model 4 + waist circumference, Model 6: model 5 + CRP, pre-transplant kidney disease diagnosis, statin use, daily prednisolone dose, Model 7: model 6 + daily fruit intake and daily vegetable intake.
Figure 3:
Hazard ratios for incident death-censored late graft failure per doubling of fasting apoB48 concentrations stratified by relevant baseline characteristics, Hazard ratios were determined by multivariate Cox proportional hazards regression analysis adjusted for age and sex. The p value for the interaction was calculated by the log rank test. eGFR, estimated glomerular filtration rate; TX, transplantation; HbA1c, glycated hemoglobin; Apo A-I, apolipoprotein A-I; Apo B, apolipoprotein B.
Figure 4:
The prospective association between continuous fasting plasma apoB48 levels and death-censored late graft failure given as adjusted hazard ratios (aHR).
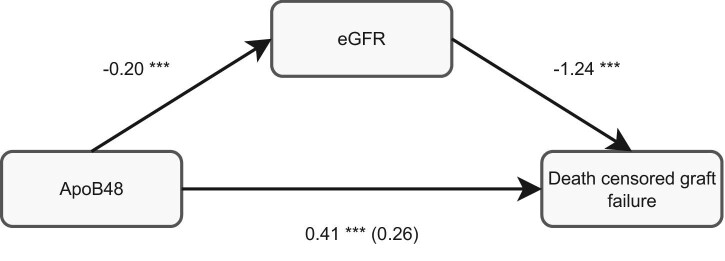
Figure 5:
Kidney function measured by eGFR is a full mediator between apoB48 and death-censored late graft failure, The odds ratio (OR) between apoB48 and death-censored graft failure was determined by logistic regression (OR, 0.41, P < .001). Adding eGFR to this model resulted in a considerably lower OR and a not significant P value (OR, 0.26, P = .066). The regression coefficient between apoB48 and eGFR was determined by linear regression (standardized β, −0.20, P < .001). Last, the OR between eGFR and death-censored late graft failure was obtained (OR, −1.24, P < .001)., ***P < .001.
ApoB48 and incident cardiovascular events and overall mortality
Cardiovascular events and overall mortality were recorded during follow-up. During a median follow-up of 5.2 [4.5–5.7] years, 74 (15.5%) KTR experienced cardiovascular events. The rate of all-cause mortality during the study period was 143 (29.7%). However, neither incident cardiovascular events [crude model, HR, 1.05 (0.85, 1.31), P = .644], nor overall mortality [crude model, odds ratio, 1.13 (0.93, 1.36), P = .211] were significantly associated with baseline apoB48 levels.
DISCUSSION
The results of this study demonstrate that baseline fasting apoB48 levels are associated with death-censored late graft failure after kidney transplantation independent of several potential confounders.
In humans, apoB48 is exclusively expressed in enterocytes where RNA editing of the apoB100 transcript takes place. Thus, fasting apoB48 in plasma is derived from incompletely lipolyzed triglyceride-rich chylomicrons formed in the absorptive state [9]. Chylomicrons transport dietary fats, cholesterol, fat-soluble nutrients and vitamins [17]. Although data comparing apoB48-only with apoB100-only mice suggested that apoB48 might be less atherogenic in experimental setting where no remnants are formed [18], this was not the case on the apoE knockout background: an experimental model of remnant lipoproteins [19]. Additional results from animal and human studies confirmed that apoB48-containing lipoproteins contribute to atherosclerotic plaque formation. In rabbits infused with LDL and chylomicron remnants, cholesterol uptake into the plaque mainly occurred from apoB48-containing chylomicron remnants [20]. Further, ApoB48 has actually been detected in human atherosclerotic plaques [21, 22]. Allograft vasculopathy resembles atherosclerotic plaque formation [6], lending biological plausibility to the results of the current study. Similarly to apoB48, the ratio between apoB48 and triglycerides was also strongly associated to graft failure during follow-up. Although not specific for apoB48-containing lipoproteins, since triglycerides are also transported in other, apoB100-containing lipoproteins, this finding could indicate that preferentially smaller chylomicron remnants are associated with late graft failure. However, more experimentation with differential isolation of apoB-containing lipoprotein subclasses followed by sizing, e.g. using nuclear magnetic resonance spectroscopy will be needed to confirm such a hypothesis. Such studies are technically challenging and conceivably require considerable amounts of plasma samples and are thus beyond the scope of the current work. Of note, no statistically significant association was observed between incident cardiovascular events or all-cause mortality and apoB48 during the study period.
Another interesting result was the strong cross-sectional association of fasting apoB48 levels with kidney function. It was previously demonstrated that individuals with lower eGFR or higher proteinuria had increased fasting circulating apoB48 concentrations [23]. Additional studies showed that the presence of CKD increased the lipemic response to a meal with a significantly higher area under the curve for triglycerides and apoB48, compared with controls [24]. Mechanistically, these findings were related, at least partly, to an increase in circulating levels of apoC-III, an endogenous major inhibitor of LPL [24, 25]. Decreased lipolysis of apoB48-containing lipoproteins likely delays their catabolic rate either directly or via competition for cellular uptake with apoB100-containing lipoproteins, whose catabolism was also significantly slowed in participants with decreased kidney function [25]. Combined, these data hint toward communication between the kidney and either the liver or the intestine, which is as of yet still only beginning to be explored.
Late graft failure is a severe long-term complication of kidney transplantation [26]. It is strongly associated with increased morbidity and mortality, as well as a drastic reduction in quality of life [5, 26]. The cost of each failed allograft in the USA approximates to $78 079 and on average results in the loss of 1.66 quality-adjusted life years [27]. Therefore, early personalized strategies for prevention but also therapeutic intervention are needed. Our data establish the prospective association between apoB48 and death-censored graft failure and thereby lend clinical meaning to this parameter. Further we show that a very large proportion of KTR already have substantially increased circulating fasting apoB48 levels when compared to healthy individuals, namely according to our estimation >85% of the participants of our study. Why this is the case is currently unclear. Conceivably, the underlying mechanisms involve certain specifics of these patients with contributions from both reduced kidney graft function as discussed above and immunosuppressive medication. However, these data clearly indicate a need to evaluate potential therapeutic intervention options. Lifestyle changes such as avoiding sugar-sweetened beverages or a high glucose intake in general have a clear pathophysiological rationale, since oral glucose mobilizes triglyceride stores from the intestine [28]. Regarding pharmacotherapy statins are recommended in KTR [29]. A potential effect of statins on chylomicron secretion will at best be minor, although a certain impact on clearance by the liver and thereby an absolute lowering effect is conceivable. In support of this reasoning, after 4 weeks of cerivastatin a significant decrease in postprandial plasma apoB48 levels was noted in T2DM patients [30]. Further, simvastatin significantly lowered fasting very large triglyceride-containing lipoprotein levels in a small study, where apoB48 levels were not determined [11], and atorvastatin reduced plasma apoB48 in healthy men [31]. In KTR no such data have been generated, to the best of our knowledge. However, our results also do not support a major impact of statin therapy on apoB48 levels. Alternatively, high levels of apoB48 containing lipoproteins could e.g. effectively be treated by oral administration of omega 3 fatty acids or established medications such as ezetimibe or fibrates [11, 12, 13]. Omega 3 fatty acids decrease chylomicron-associated triglyceride levels by lowering the production rate of apoB-containing lipoproteins through stimulating post-translational apoB degradation [32] as well as by increasing the catabolic rate of triglyceride-rich lipoproteins via an increase in LPL activity [12]. Ezetimibe blocks the intestinal Niemann-Pick C1-like 1 protein transporter, thereby reducing intestinal cholesterol absorption with a subsequent decrease in circulating fasting as well as postprandial triglyceride-rich [33] or specifically fasting remnant lipoproteins [11]. Fibrates, pemafibrate in particular, have been shown to lower circulating apoB48 levels by ∼55%, which could be due to direct transcriptional effects on enterocytes [34] or a decrease in plasma apoC-III [35]. However, next to fibrates being suspected to have at least some nephrotoxic potential, they have no effect on incident cardiovascular events, a notion that has resulted in diminished clinical interest in this class of drugs [35]. Further, novel therapeutic intervention strategies such as antisense oligonucleotides targeting hepatic apoC-III or ANGTL3, another endogenous LPL inhibitor, expression specifically and directly might also have a future role for treating severe cases of remnant-associated hypertriglyceridemia in KTR [36]. Additionally, apoB48 levels may also be considered when evaluating maintenance immunosuppressive drugs and their differential metabolic impact.
The TransplantLines Nutritional Biobank and Cohort study represents a large, well-characterized KTR cohort. However, it is from a single center and most KTR are from a northern/western European background. Therefore, the presented results should ideally be replicated in more ethnically diverse populations as well. Since data collection was carried out between August 2001 and July 2003, the main calcineurin inhibitor used was cyclosporine. This does not fully reflect the current standard of care, which is the predominant prescription of tacrolimus. Compared with cyclosporine tacrolimus is believed to have a more favorable metabolic profile [37]. Corticosteroid doses were also relatively high in our medical center, which might affect lipid metabolism [38]. Additionally, it would be desirable to have a means to specifically infer also apoB100 levels from the conducted measurements, e.g. by subtracting apoB48 concentrations from total apoB levels. However, this is unfortunately in the present work not possible due to the use of two different assay systems. International switching units and cumulative prednisolone doses were not recorded in the current study. Further studies investigating longitudinal apoB48 concentrations and their association to immunosuppressive units (ISU) and different types of immunosuppressive medication are therefore desirable. In vitro models including but not limited to intestinal organoid cultures could help to establish molecular mechanisms and dose-response relationships between different immunosuppressive medications and apoB48 expression. Due to the observational design of the study, residual confounding cannot be ruled out and mechanistic conclusions regarding the prospective association between apoB48 and graft failure cannot be drawn. Therefore, future experimental research should be focused on establishing such a mechanistic link, which ideally should also consider the metabolic effect of immunosuppressive medication use on apoB48 concentrations. Further, experimental models would offer the chance to explore the extent to which a decline in kidney function or an acutely absent renal function impact gene expression of apoB48 in the small intestine and subsequently the secretion of chylomicrons. In addition, the association between longitudinal changes in apoB48 levels and long-term transplant outcomes such as graft failure would be interesting to investigate in KTR. Overall, exploring alternative maintenance immunosuppression strategies and safe long-term co-medications with the aim to limit cardiometabolic side effects, including apoB48, remains a long-term goal in transplantation medicine that future research needs to focus on.
In conclusion, baseline fasting apoB48 levels predict death-censored kidney graft failure after kidney transplantation independent of several potential confounders. These results underline the importance of considering intestinal lipoprotein metabolism in KTR. As a potential clinical implication our data provide a rationale for determining apoB48 levels in KTR and for considering pharmacological interventions aimed at decreasing intestinal lipid absorption and circulating levels of intestine-derived remnant lipoproteins to improve long-term outcomes of kidney transplantation.
Supplementary Material
Contributor Information
Tamas Szili-Torok, Department of Internal Medicine, University Medical Center Groningen, University of Groningen, Groningen, the Netherlands.
Martin H de Borst, Department of Internal Medicine, University Medical Center Groningen, University of Groningen, Groningen, the Netherlands.
Alexandra Soteriou, Department of Internal Medicine, University Medical Center Groningen, University of Groningen, Groningen, the Netherlands.
Laura Post, Department of Internal Medicine, University Medical Center Groningen, University of Groningen, Groningen, the Netherlands.
Stephan J L Bakker, Department of Internal Medicine, University Medical Center Groningen, University of Groningen, Groningen, the Netherlands.
Uwe J F Tietge, Division of Clinical Chemistry, Department of Laboratory Medicine, Karolinska Institutet, Stockholm, Sweden; Clinical Chemistry, Karolinska University Laboratory, Karolinska University Hospital, Stockholm, Sweden.
DATA AVAILABILITY STATEMENT
The data underlying this article will be shared on reasonable request to the corresponding author.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1. Thurlow JS, Joshi M, Yan G et al. Global epidemiology of end-stage kidney disease and disparities in kidney replacement therapy. Am J Nephrol 2021;52:98–107. 10.1159/000514550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lemoine M, Titeca Beauport D, Lobbedez T et al. Risk factors for early graft failure and death after kidney transplantation in recipients older than 70 years. Kidney Int Rep 2019;4:656–66. 10.1016/j.ekir.2019.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Josephson MA, Becker Y, Budde K et al. Challenges in the management of the kidney allograft: from decline to failure: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) controversies conference. Kidney Int 2023; 104:1076–91. 10.1016/j.kint.2023.05.010 [DOI] [PubMed] [Google Scholar]
- 4. Lubetzky M, Tantisattamo E, Molnar MZ et al. The failing kidney allograft: a review and recommendations for the care and management of a complex group of patients. Am J Transplant 2021;21:2937–49. 10.1111/ajt.16717 [DOI] [PubMed] [Google Scholar]
- 5. Howell M, Wong G, Rose J et al. Patient preferences for outcomes after kidney transplantation: a best-worst scaling survey. Transplantation 2017;101:2765–73. 10.1097/TP.0000000000001793 [DOI] [PubMed] [Google Scholar]
- 6. Merola J, Jane-Wit DD, Pober JS. Recent advances in allograft vasculopathy. Curr Opin Organ Transplant 2017;22:1–7. 10.1097/MOT.0000000000000370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Anderson JLC, Poot ML, Steffen HLM et al. The Framingham Risk Score is associated with chronic graft failure in renal transplant recipients. J Clin Med 2021;10:3287. 10.3390/jcm10153287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nordestgaard BG. Triglyceride-rich lipoproteins and atherosclerotic cardiovascular disease: new insights from epidemiology, genetics, and biology. Circ Res 2016;118:547–63. 10.1161/CIRCRESAHA.115.306249 [DOI] [PubMed] [Google Scholar]
- 9. Mehta A, Shapiro MD. Apolipoproteins in vascular biology and atherosclerotic disease. Nat Rev Cardiol 2022;19:168–79. 10.1038/s41569-021-00613-5 [DOI] [PubMed] [Google Scholar]
- 10. Anderson JLC, Bakker SJL, Tietge UJF. The triglyceride to HDL-cholesterol ratio and chronic graft failure in renal transplantation. J Clin Lipidol 2021;15:301–10. 10.1016/j.jacl.2021.01.009 [DOI] [PubMed] [Google Scholar]
- 11. Ahmed O, Littmann K, Gustafsson U et al. Ezetimibe in combination with simvastatin reduces remnant cholesterol without affecting biliary lipid concentrations in gallstone patients. J Am Heart Assoc 2018;7:e009876. 10.1161/JAHA.118.009876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Park Y, Harris WS. Omega-3 fatty acid supplementation accelerates chylomicron triglyceride clearance. J Lipid Res 2003;44:455–63. 10.1194/jlr.M200282-JLR200 [DOI] [PubMed] [Google Scholar]
- 13. Araki E, Yamashita S, Arai H et al. Efficacy and safety of pemafibrate in people with type 2 diabetes and elevated triglyceride levels: 52-week data from the PROVIDE study. Diabetes Obes Metab 2019;21:1737–44. 10.1111/dom.13686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Szili-Torok T, Annema W, Anderson JLC et al. HDL cholesterol efflux predicts incident new-onset diabetes after transplantation (NODAT) in renal transplant recipients independent of HDL cholesterol levels. Diabetes 2019;68:1915–23. 10.2337/db18-1267 [DOI] [PubMed] [Google Scholar]
- 15. de Boer JF, Bahr MJ, Böker KH et al. Plasma levels of PBEF/Nampt/visfatin are decreased in patients with liver cirrhosis. Am J Physiol Gastrointest Liver Physiol 2009;296:G196–201. 10.1152/ajpgi.00029.2008 [DOI] [PubMed] [Google Scholar]
- 16. White SL, Polkinghorne KR, Atkins RC et al. Comparison of the prevalence and mortality risk of CKD in Australia using the CKD Epidemiology Collaboration (CKD-EPI) and Modification of Diet in Renal Disease (MDRD) study GFR estimating equations: the AusDiab (Australian Diabetes, Obesity and Lifestyle) Study. Am J Kidney Dis 2010;55:660–70. [DOI] [PubMed] [Google Scholar]
- 17. Tomkin GH, Owens D. The chylomicron: relationship to atherosclerosis. Int J Vasc Med 2012;2012:784536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Véniant MM, Zlot CH, Walzem RL et al. Lipoprotein clearance mechanisms in LDL receptor-deficient “apo-B48-only” and “apo-B100-only” mice. J Clin Invest 1998;102:1559–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Véniant MM, Pierotti V, Newland D et al. Susceptibility to atherosclerosis in mice expressing exclusively apolipoprotein B48 or apolipoprotein B100. J Clin Invest 1997;100:180–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Proctor SD, Mamo JC. Intimal retention of cholesterol derived from apolipoprotein B100- and apolipoprotein B48-containing lipoproteins in carotid arteries of Watanabe heritable hyperlipidemic rabbits. Arterioscler Thromb Vasc Biol 2003;23:1595–600. 10.1161/01.ATV.0000084638.14534.0A [DOI] [PubMed] [Google Scholar]
- 21. Nakano T, Nakajima K, Niimi M et al. Detection of apolipoproteins b-48 and b-100 carrying particles in lipoprotein fractions extracted from human aortic atherosclerotic plaques in sudden cardiac death cases. Clin Chim Acta 2008;390:38–43. 10.1016/j.cca.2007.12.012 [DOI] [PubMed] [Google Scholar]
- 22. Pal S, Semorine K, Watts GF et al. Identification of lipoproteins of intestinal origin in human atherosclerotic plaque. Clin Chem Lab Med 2003;41:792–5. 10.1515/CCLM.2003.120 [DOI] [PubMed] [Google Scholar]
- 23. Okubo M, Hanada H, Matsui M et al. Serum apolipoprotein b-48 concentration is associated with a reduced estimated glomerular filtration rate and increased proteinuria. J Atheroscler Thromb 2014;21:974–82. 10.5551/jat.23309 [DOI] [PubMed] [Google Scholar]
- 24. Saland JM, Satlin LM, Zalsos-Johnson J et al. Impaired postprandial lipemic response in chronic kidney disease. Kidney Int 2016;90:172–80. 10.1016/j.kint.2016.02.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chan DT, Dogra GK, Irish AB et al. Chronic kidney disease delays VLDL-apoB-100 particle catabolism: potential role of apolipoprotein c-III. J Lipid Res 2009;50:2524–31. 10.1194/jlr.P900003-JLR200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Van Loon E, Bernards J, Van Craenenbroeck AH et al. The causes of kidney allograft failure: more than alloimmunity. A viewpoint article. Transplantation 2020;104:e46–56. 10.1097/TP.0000000000003012 [DOI] [PubMed] [Google Scholar]
- 27. Sussell J, Silverstein AR, Goutam P et al. The economic burden of kidney graft failure in the United States. Am J Transplant 2020;20:1323–33. 10.1111/ajt.15750 [DOI] [PubMed] [Google Scholar]
- 28. Xiao C, Stahel P, Carreiro AL et al. Oral glucose mobilizes triglyceride stores from the human intestine. Cell Mol Gastroenterol Hepatol 2019;7:313–37. 10.1016/j.jcmgh.2018.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. (KDIGO) KDIGO . KDIGO clinical practice guideline for lipid management in chronic kidney disease. Chapter 2: Pharmacological cholesterol-lowering treatment in adults. Kidney Int Suppl 2013;3:271–9. 10.1038/kisup.2013.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Battula SB, Fitzsimons O, Moreno S et al. Postprandial apolipoprotein B48-and B100-containing lipoproteins in type 2 diabetes: do statins have a specific effect on triglyceride metabolism? Metabolism 2000;49:1049–54. 10.1053/meta.2000.7744 [DOI] [PubMed] [Google Scholar]
- 31. Chan DC, Watts GF, Barrett PH et al. Effect of atorvastatin on chylomicron remnant metabolism in visceral obesity: a study employing a new stable isotope breath test. J Lipid Res 2002;43:706–12. 10.1016/S0022-2275(20)30112-7 [DOI] [PubMed] [Google Scholar]
- 32. Fisher EA, Pan M, Chen X et al. The triple threat to nascent apolipoprotein b. Evidence for multiple, distinct degradative pathways. J Biol Chem 2001;276:27855–63. 10.1074/jbc.M008885200 [DOI] [PubMed] [Google Scholar]
- 33. Bozzetto L, Annuzzi G, Corte GD et al. Ezetimibe beneficially influences fasting and postprandial triglyceride-rich lipoproteins in type 2 diabetes. Atherosclerosis 2011;217:142–8. 10.1016/j.atherosclerosis.2011.03.012 [DOI] [PubMed] [Google Scholar]
- 34. Hennuyer N, Duplan I, Paquet C et al. The novel selective PPARα modulator (SPPARMα) pemafibrate improves dyslipidemia, enhances reverse cholesterol transport and decreases inflammation and atherosclerosis. Atherosclerosis 2016;249:200–8. 10.1016/j.atherosclerosis.2016.03.003 [DOI] [PubMed] [Google Scholar]
- 35. Das Pradhan A, Glynn RJ, Fruchart JC et al. Triglyceride lowering with pemafibrate to reduce cardiovascular risk. N Engl J Med 2022;387:1923–34. 10.1056/NEJMoa2210645 [DOI] [PubMed] [Google Scholar]
- 36. Ginsberg HN, Goldberg IJ. Broadening the scope of dyslipidemia therapy by targeting APOC3 (apolipoprotein C3) and ANGPTL3 (angiopoietin-like protein 3). Arterioscler Thromb Vasc Biol 2023;43:388–98. 10.1161/ATVBAHA.122.317966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. McCune TR, Thacker ILR, Peters TG et al. Effects of tacrolimus on hyperlipidemia after successful renal transplantation: a southeastern organ procurement foundation multicenter clinical study. Transplantation 1998;65:87–92. 10.1097/00007890-199801150-00017 [DOI] [PubMed] [Google Scholar]
- 38. Miller M, Stone NJ, Ballantyne C et al. Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation 2011;123:2292–333. 10.1161/CIR.0b013e3182160726 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.