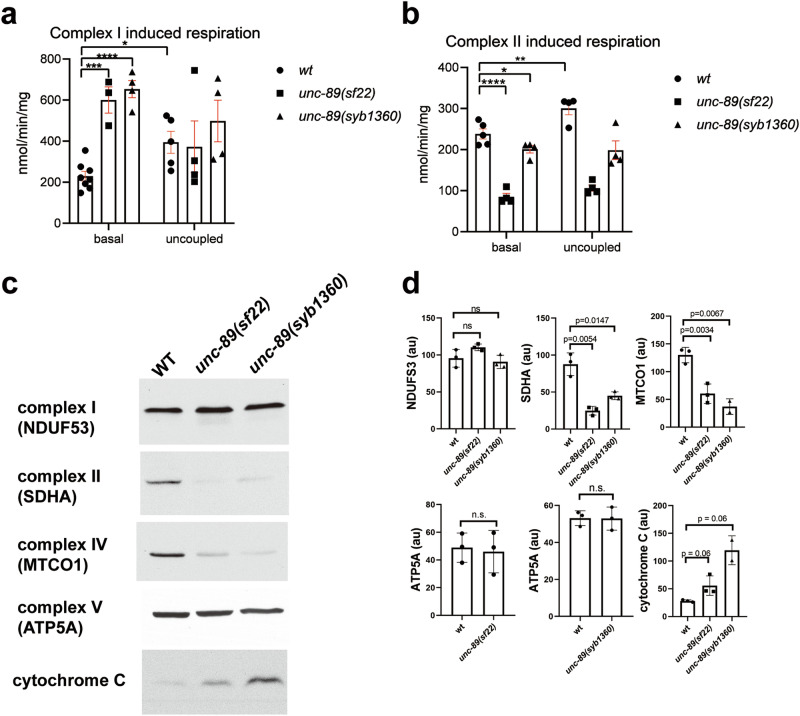
Fig. 8. PK2 KtoA mutants display altered mitochondrial respiration and altered levels of some electron transport chain complexes.
a, b Muscle mitochondria from PK2 KtoA mutants have increased complex I and decreased complex II basal respiration and each cannot be uncoupled. a Complex I (glutamate and malate) stimulated basal and uncoupled mitochondrial oxygen consumption rates measured in isolated muscle mitochondria from wild type (wt), unc-89(sf22), and unc-89 (syb1360) mutant strains (n = 3–8 per strain). b Complex II (succinate) stimulated basal and uncoupled mitochondrial oxygen consumption rates measured in isolated muscle mitochondria from wild type (wt), unc-89(sf22), and unc-89 (syb1360) mutant strains (n = 4–5 per strain). Means and standard errors of the mean are represented. Significance was tested by one-way ANOVA followed by Tukey’s post hoc test. *p ≤ 0.05, **p ≤ 0.005, ***p ≤ 0.0005, ****p ≤ 0.00005. c, d PK2 KtoA mutants have altered levels of electron transport chain complexes. c Representative western blots reacted against antibodies to the indicated components of complexes I, II, IV, V, and cytochrome C. d Quantitation of these results with n = 3. au: arbitrary units. Statistical significance was assessed using an unpaired t-test with Welch’s correction. Note that as compared to wild type, complexes I and V are normal, but complexes II and IV are reduced, and cytochrome C is elevated in the PK2 KtoA mutants.