Abstract
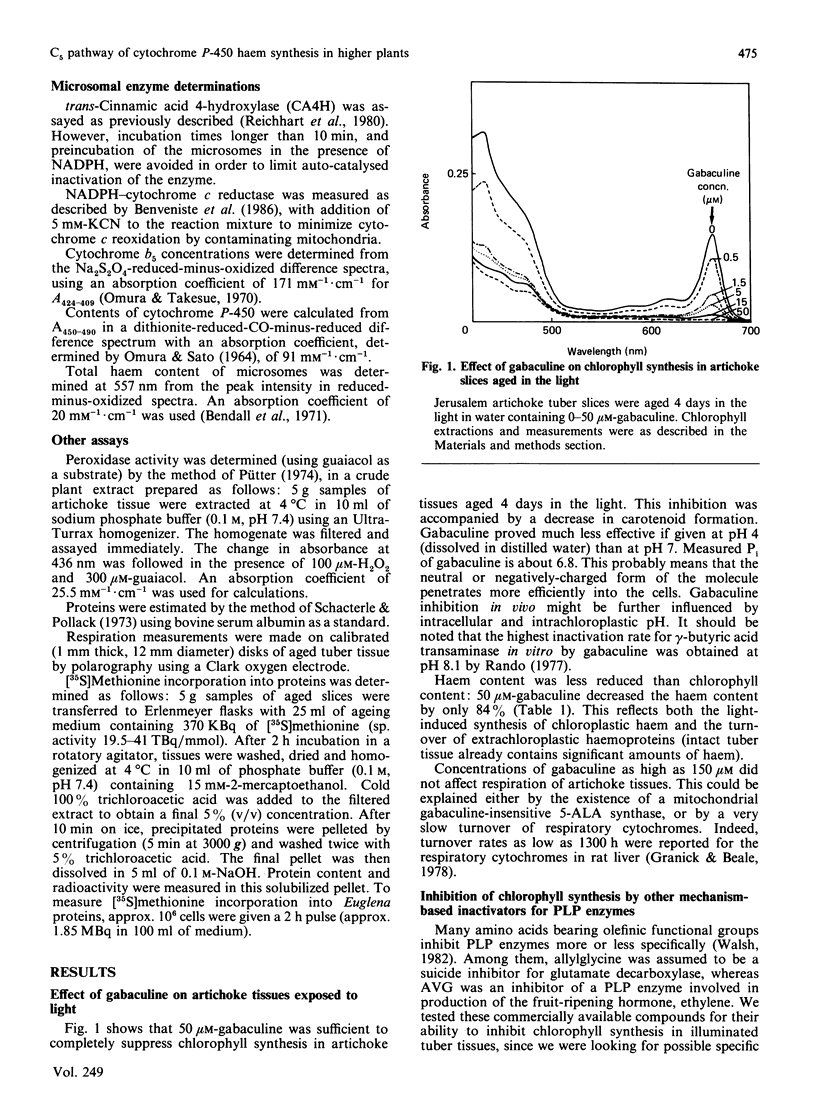
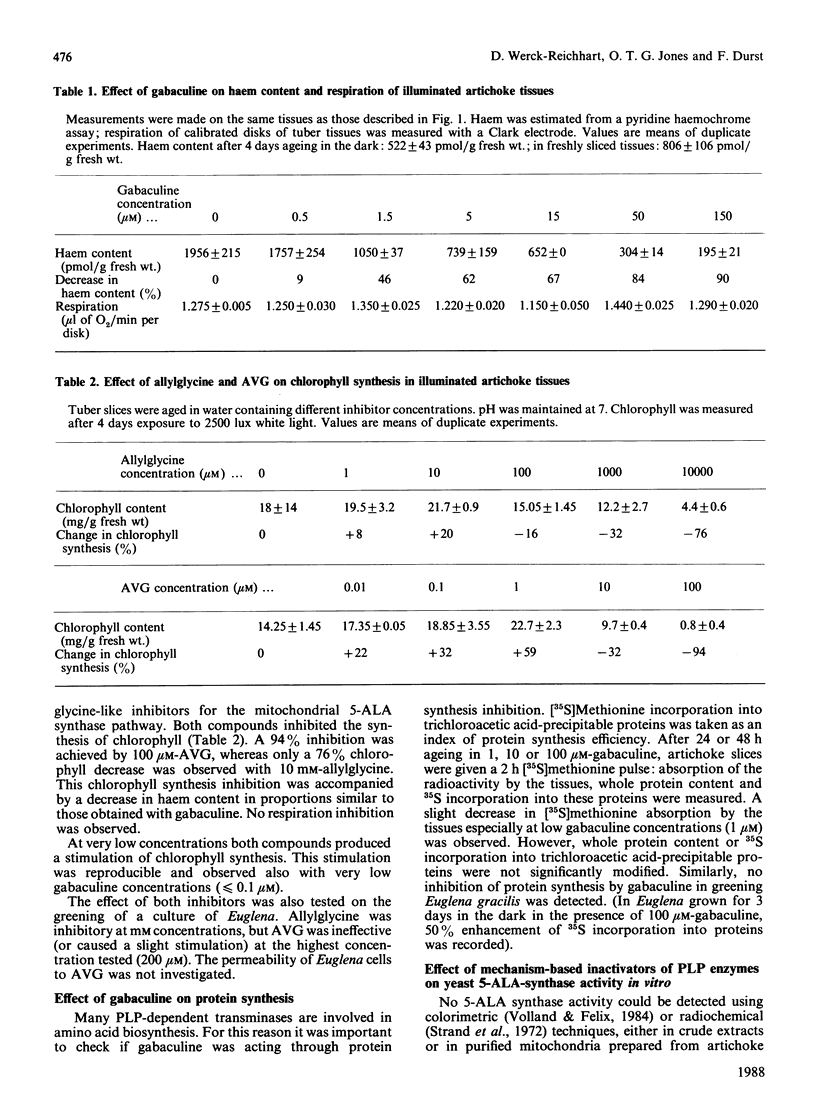
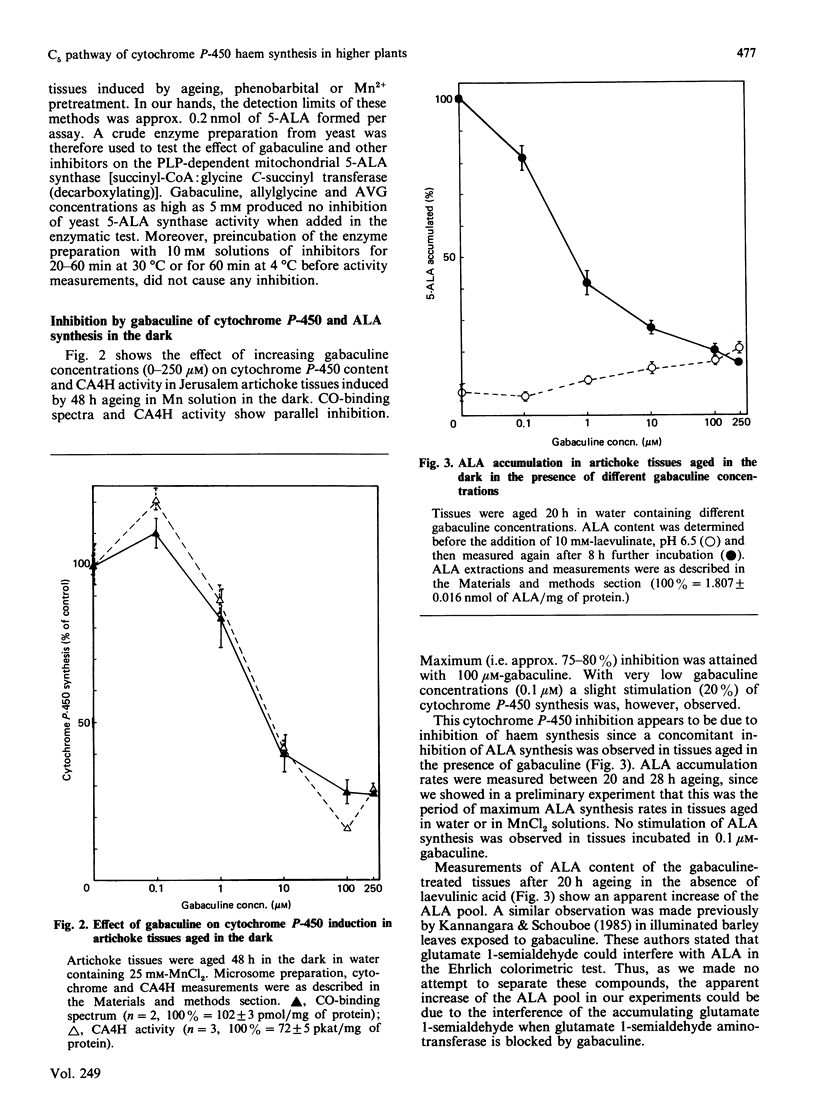
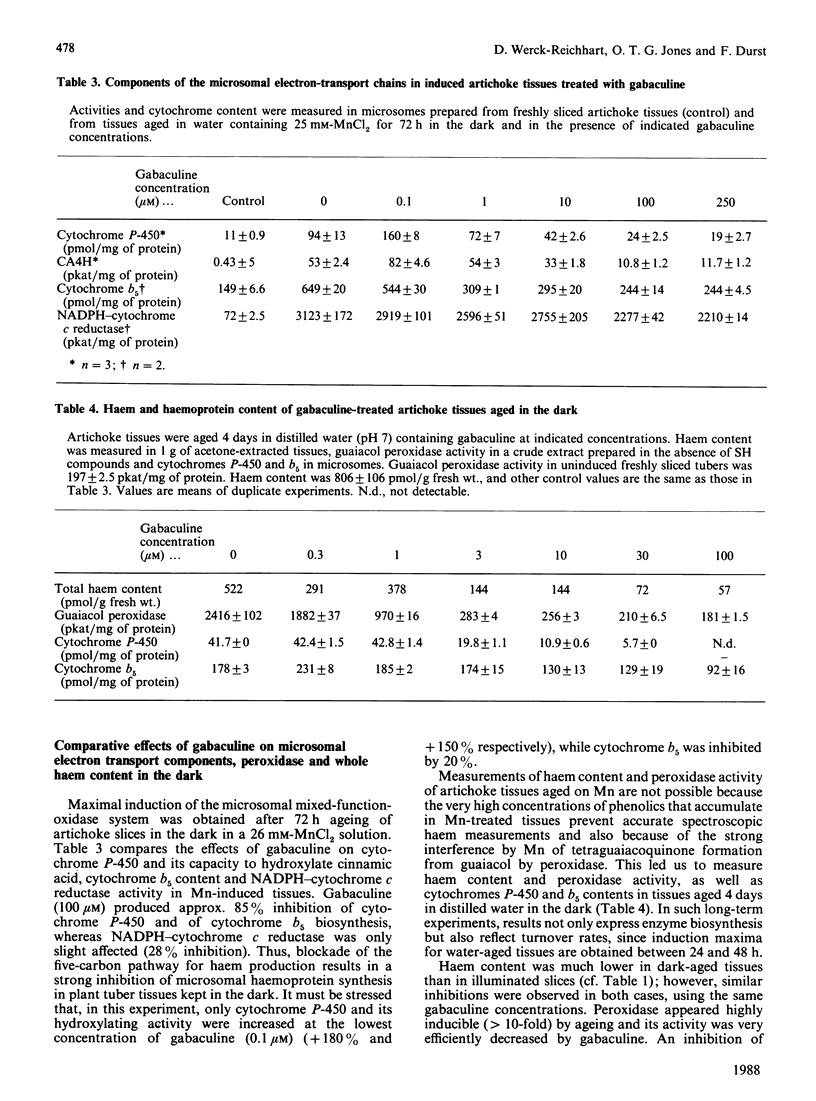
Chlorophyll and haem synthesis in illuminated Jerusalem artichoke tuber tissues were very efficiently inhibited by gabaculine (3-amino-2,3-dihydrobenzoic acid). This inhibition seems to be due specifically to a blockade of the pathway for 5-aminolaevulinate biosynthesis which used glutamate as a substrate (the so-called C5 pathway) since we could not detect any inhibition of protein synthesis in the treated tissues and there was no effect of gabaculine on the glycine-dependent yeast 5-aminolaevulinate synthase used as a model. In dark-aged artichoke tissues, gabaculine also effectively blocked cytochrome P-450 induction, peroxidase activity and 5-aminolaevulinic acid synthesis, thus suggesting the involvement of a C5 pathway in cytoplasmic and microsomal haemoprotein synthesis in this higher plant. Allylglycine and (2-amino-ethyloxyvinyl)glycine, two olefinic glycine analogues which are potential suicide inhibitors of pyridoxal phosphate enzymes, were also demonstrated to be effective blockers of chlorophyll synthesis in artichoke tuber and Euglena cells exposed to light.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beale S. I., Foley T., Dzelzkalns V. delta-Aminolevulinic acid synthase from Euglena gracilis. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1666–1669. doi: 10.1073/pnas.78.3.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beale S. I. The biosynthesis of delta-aminolevulinic acid in Chlorella. Plant Physiol. 1970 Apr;45(4):504–506. doi: 10.1104/pp.45.4.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste I., Gabriac B., Durst F. Purification and characterization of the NADPH-cytochrome P-450 (cytochrome c) reductase from higher-plant microsomal fraction. Biochem J. 1986 Apr 15;235(2):365–373. doi: 10.1042/bj2350365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett G., Yonaha K., Toyama S., Soda K., Walsh C. Studies on the kinetics and stoichiometry of inactivation of Pseudomonas omega-amino acid:pyruvate transaminase by gabaculine. J Biol Chem. 1980 Jan 25;255(2):428–432. [PubMed] [Google Scholar]
- Castelfranco P. A., Jones O. T. Protoheme turnover and chlorophyll synthesis in greening barley tissue. Plant Physiol. 1975 Mar;55(3):485–490. doi: 10.1104/pp.55.3.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser R., Hauri H. P., Meyer U. A. The turnover of cytochrome P450b. FEBS Lett. 1982 Oct 18;147(2):239–242. doi: 10.1016/0014-5793(82)81050-8. [DOI] [PubMed] [Google Scholar]
- Granick S., Beale S. I. Hemes, chlorophylls, and related compounds: biosynthesis and metabolic regulation. Adv Enzymol Relat Areas Mol Biol. 1978;46:33–203. doi: 10.1002/9780470122914.ch2. [DOI] [PubMed] [Google Scholar]
- MAUZERALL D., GRANICK S. The occurrence and determination of delta-amino-levulinic acid and porphobilinogen in urine. J Biol Chem. 1956 Mar;219(1):435–446. [PubMed] [Google Scholar]
- Nandi D. L., Shemin D. Delta-aminolevulinic acid dehydratase of Rhodopseudomonas spheroides. 3. Mechanism of porphobilinogen synthesis. J Biol Chem. 1968 Mar 25;243(6):1236–1242. [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. I. EVIDENCE FOR ITS HEMOPROTEIN NATURE. J Biol Chem. 1964 Jul;239:2370–2378. [PubMed] [Google Scholar]
- Omura T., Takesue S. A new method for simultaneous purification of cytochrome b5 and NADPH-cytochrome c reductase from rat liver microsomes. J Biochem. 1970 Feb;67(2):249–257. doi: 10.1093/oxfordjournals.jbchem.a129248. [DOI] [PubMed] [Google Scholar]
- Parkinson A., Thomas P. E., Ryan D. E., Levin W. The in vivo turnover of rat liver microsomal epoxide hydrolase and both the apoprotein and heme moieties of specific cytochrome P-450 isozymes. Arch Biochem Biophys. 1983 Aug;225(1):216–236. doi: 10.1016/0003-9861(83)90025-5. [DOI] [PubMed] [Google Scholar]
- Porra R. J. Labelling of chlorophylls and precursors by [2-14C]glycine and 2-[1-14C]oxoglutarate in Rhodopseudomonas spheroides and Zea mays. Resolution of the C5 and Shemin pathways of 5-aminolaevulinate biosynthesis by thin-layer radiochromatography. Eur J Biochem. 1986 Apr 1;156(1):111–121. doi: 10.1111/j.1432-1033.1986.tb09555.x. [DOI] [PubMed] [Google Scholar]
- Ramaswamy N. K., Nair P. M. Pathway for the biosynthesis of delta-aminolevulinic acid in greening potatoes. Indian J Biochem Biophys. 1976 Dec;13(4):394–397. [PubMed] [Google Scholar]
- Rando R. R. Mechanism of the irreversible inhibition of gamma-aminobutyric acid-alpha-ketoglutaric acid transaminase by the neutrotoxin gabaculine. Biochemistry. 1977 Oct 18;16(21):4604–4610. doi: 10.1021/bi00640a012. [DOI] [PubMed] [Google Scholar]
- Reichhart D., Salaün J. P., Benveniste I., Durst F. Time Course of Induction of Cytochrome P-450, NADPH-Cytochrome c Reductase, and Cinnamic Acid Hydroxylase by Phenobarbital, Ethanol, Herbicides, and Manganese in Higher Plant Microsomes. Plant Physiol. 1980 Oct;66(4):600–604. doi: 10.1104/pp.66.4.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadano H., Omura T. Reversible transfer of heme between different molecular species of microsome-bound cytochrome P-450 in rat liver. Biochem Biophys Res Commun. 1983 Nov 15;116(3):1013–1019. doi: 10.1016/s0006-291x(83)80243-5. [DOI] [PubMed] [Google Scholar]
- Sadano H., Omura T. Turnover of two drug-inducible forms of microsomal cytochrome P-450 in rat liver. J Biochem. 1983 May;93(5):1375–1383. doi: 10.1093/oxfordjournals.jbchem.a134272. [DOI] [PubMed] [Google Scholar]
- Schacterle G. R., Pollack R. L. A simplified method for the quantitative assay of small amounts of protein in biologic material. Anal Biochem. 1973 Feb;51(2):654–655. doi: 10.1016/0003-2697(73)90523-x. [DOI] [PubMed] [Google Scholar]
- Schön A., Krupp G., Gough S., Berry-Lowe S., Kannangara C. G., Söll D. The RNA required in the first step of chlorophyll biosynthesis is a chloroplast glutamate tRNA. Nature. 1986 Jul 17;322(6076):281–284. doi: 10.1038/322281a0. [DOI] [PubMed] [Google Scholar]
- Soper T. S., Manning J. M. Inactivation of pyridoxal phosphate enzymes by gabaculine. Correlation with enzymic exchange of beta-protons. J Biol Chem. 1982 Dec 10;257(23):13930–13936. [PubMed] [Google Scholar]
- Strand L. J., Swanson A. L., Manning J., Branch S., Marver H. S. Radiochemical microassay of delta-aminolevulinic acid synthetase in hepatic and erythroid tissues. Anal Biochem. 1972 Jun;47(2):457–470. doi: 10.1016/0003-2697(72)90139-x. [DOI] [PubMed] [Google Scholar]
- Volland C., Felix F. Isolation and properties of 5-aminolevulinate synthase from the yeast Saccharomyces cerevisiae. Eur J Biochem. 1984 Aug 1;142(3):551–557. doi: 10.1111/j.1432-1033.1984.tb08321.x. [DOI] [PubMed] [Google Scholar]
- Weinstein J. D., Beale S. I. Separate physiological roles and subcellular compartments for two tetrapyrrole biosynthetic pathways in Euglena gracilis. J Biol Chem. 1983 Jun 10;258(11):6799–6807. [PubMed] [Google Scholar]


