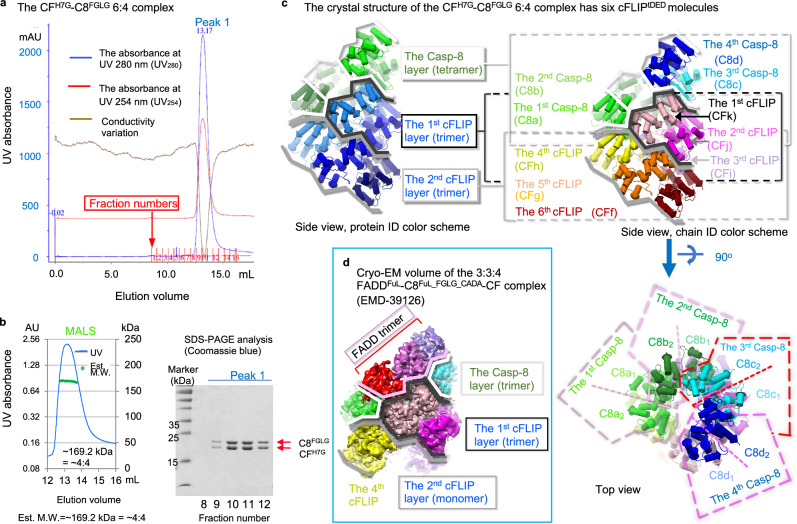
Fig. 1. CFH7G and C8FGLG form a binary oligomeric complex in solution and crystals.
a, b SEC-MALS analysis of the binary CFH7G-C8FGLG complex, with corresponding SDS-PAGE analysis in (b) for the peak fractions in (a). These results demonstrate that cFLIPtDED and Casp-8tDED form an oligomeric complex in solution. The MALS data suggests that the complex is smaller in solution than in crystals, indicating that the end molecules, likely cFLIPtDED, have high dissociation constants similar to the DD complex58. Est. M.W., estimated molecular weight. The experiments were repeated twice with similar results. Source data are provided as a Source Data file. c The pipes-and-planks diagrams illustrate the crystal structure of the binary CFH7G-C8FGLG complex from different perspectives. In the protein ID color scheme of Figs. 1–3, Casp-8 and cFLIP are colored in different shades of green and blue, respectively, while in the chain ID color scheme, the molecules in Figs. 1–3 are colored as their counterparts in Fig. 3g. Additionally, the C8FGLG layer/tetramer, the 1st CFH7G layer/trimer, and the 2nd CFH7G layer/trimer are consistently highlighted by white, dark gray, and gray lines, respectively. C8d1 and C8d2 denote DED1 and DED2 of Casp-8tDED chain d, respectively, while CFf represents cFLIPtDED chain f. The dashed boxes in the top view indicate the positions of Casp-8 molecules as well as the DED1 and DED2 domains. d Depicts the cryo-EM volume of our previous ternary 3:3:4 FADDFuL-C8FuL_FGLG_CADA-CF complex for comparison (EMDB: EMD-39126)56. The FADD trimer is highlighted by pink lines, while the C8FGLG trimer is highlighted by white lines.