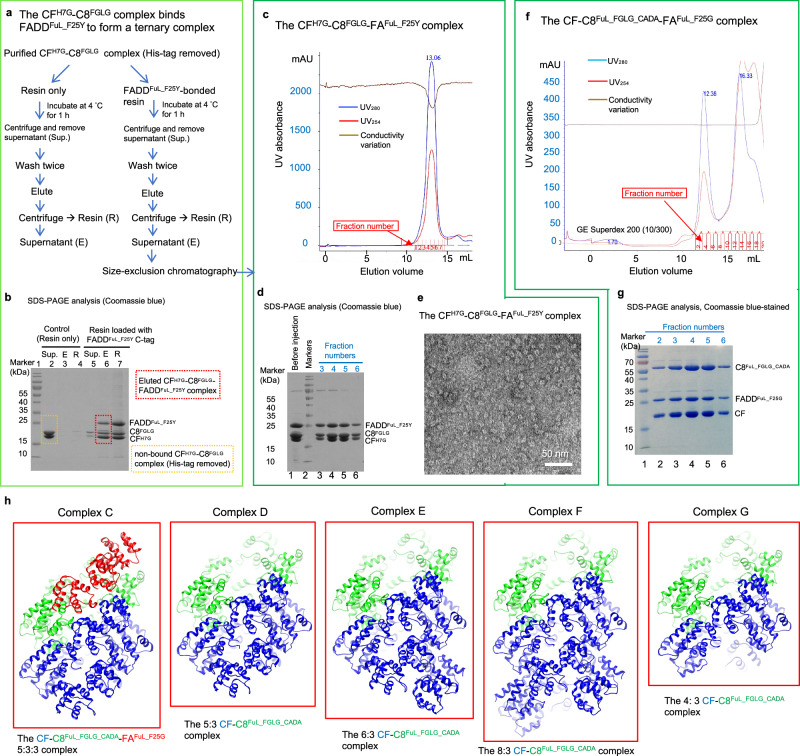
Fig. 5. The binary CFH7G-C8FGLG complex binds FADD to form the ternary complex in reverse order.
a Procedure of reconstituting the ternary CFH7G-C8FGLG-FAFuL_F25Y complex in reverse order, utilizing resin-bound FADDFuL_F25Y to pulldown tag-removed binary CFH7G-C8FGLG complex, with resin-only serving as a negative control. b SDS-PAGE analysis of the unbound supernatant fraction (Sup.), eluted bound protein fraction (E), and resin after elution (R) from (a). c Gel filtration profile of the ternary CFH7G-C8FGLG-FAFuL_F25Y complex, using the sample from the eluted bound protein fraction (E) in (a) and (b), analyzed with a Superdex 200 increase (10/300 GL) column. d SDS-PAGE analysis of the peak fractions from (c). e Negative-stain electron microscopy (EM) analysis of the peak fractions in (c), demonstrating the globular shape of the ternary CFH7G-C8FGLG-FAFuL_F25Y complex obtained in reverse order. Scale bar = 50 nm. f Gel filtration profile of the CFH7G-C8FuL_FGLG_CADA-FAFuL_F25G complex obtained in reverse order following the procedure in (a). g SDS-PAGE analysis of the peak fractions from (f). The experiments of (b), (d), (e), and (g) were repeated twice with similar results. Source data are provided as a Source Data file. h Ribbon diagrams illustrating the atomic coordinates of five cryo-EM structures obtained from the cryo-EM sample of the CFH7G-C8FuL_FGLG_CADA-FAFuL_F25G complex sample in (f). Blue, green, and red ribbons represent cFLIP, Casp-8, and FADD, respectively.