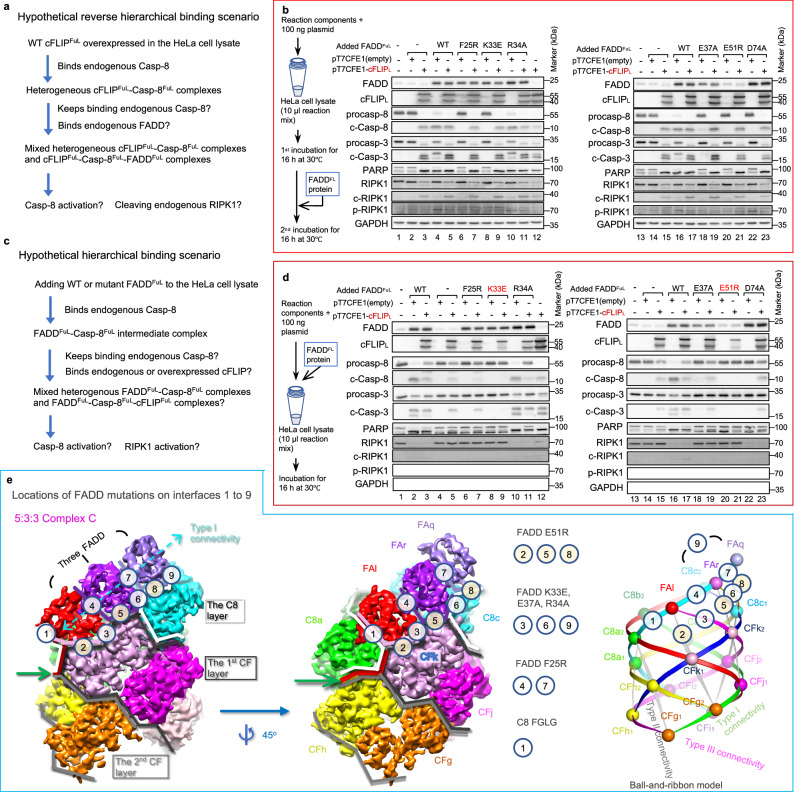
Fig. 6. Expressed cFLIP inhibits FADD-induced Casp-8 activation but partially activates Casp-8.
a Illustration of possible reactions in HeLa cell lysate under a hypothetical reverse hierarchical binding scenario. Overexpression of cFLIPFuL could trigger reverse binding by forming a binary complex with endogenous Casp-8, which can then recruit FADD. Possible biochemical reactions, demonstrated in (c) and (d), include partial Casp-8 activation and RIPK1 cleavage. b Illustration of possible reactions in HeLa cell lysate under a hypothetical hierarchical binding scenario. The addition of FADD protein could initiate the formation of an intermediate complex with endogenous Casp-8, which could then recruit either Casp-8 or cFLIP. Added FADD could also bind RIPK1. Possible biochemical reactions, demonstrated in (c) and (d), include full Casp-8 activation and RIPK1 activation. c Cell-lysate-based mutagenesis results. cFLIP-expressing or control plasmids were added to HeLa cell lysate for a 16-h incubation, followed by the addition of FADD protein for another 16-h incubation. Results were analyzed by western blotting. The western blotting data were repeated twice with similar results. d Cell-lysate-based mutagenesis results with simultaneous addition of cFLIP-expressing plasmids and FADD protein to HeLa cell lysate for a 16-h incubation. The effects of FADD mutations were examined by western blotting. Source data are provided as a Source Data file. e Illustration of seven interfaces between FADD and the binary CF-C8 sub-complex and two interfaces between adjacent FADD molecules in the triple-FADD 5:5:3 Complex C. The complex is shown as molecular surfaces using cryo-EM envelops and as a ball-and-ribbon model. Locations of various FADD mutations on different interfaces are indicated. Green arrows point to the CBS, illustrated by red lines, for recruiting Casp-8 molecule C8a. White and gray lines indicate the Casp-8 layer or cFLIP layers. Interface residues are shown in Supplementary Fig. 8b. The molecules are colored as their counterparts in Figs. 1d and 3g. C8d1 denotes DED1 of Casp-8tDED chain d, while CFf represents cFLIPtDED chain (f).