Abstract
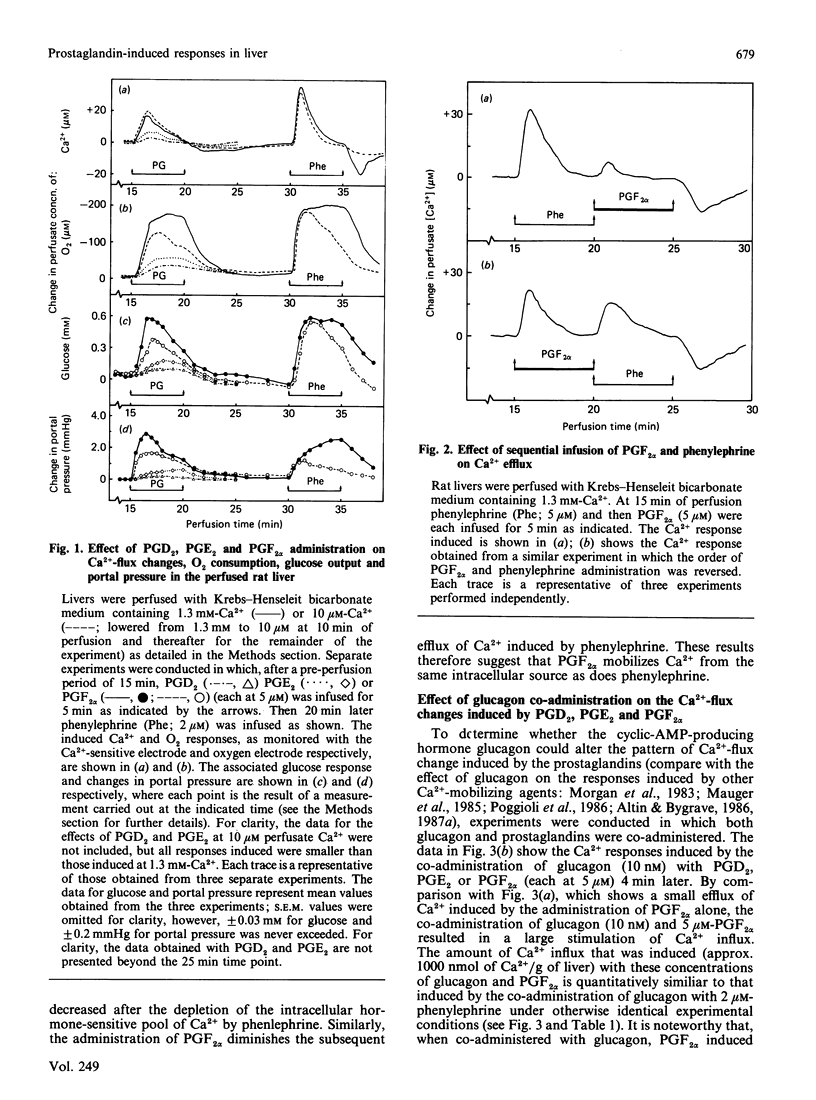
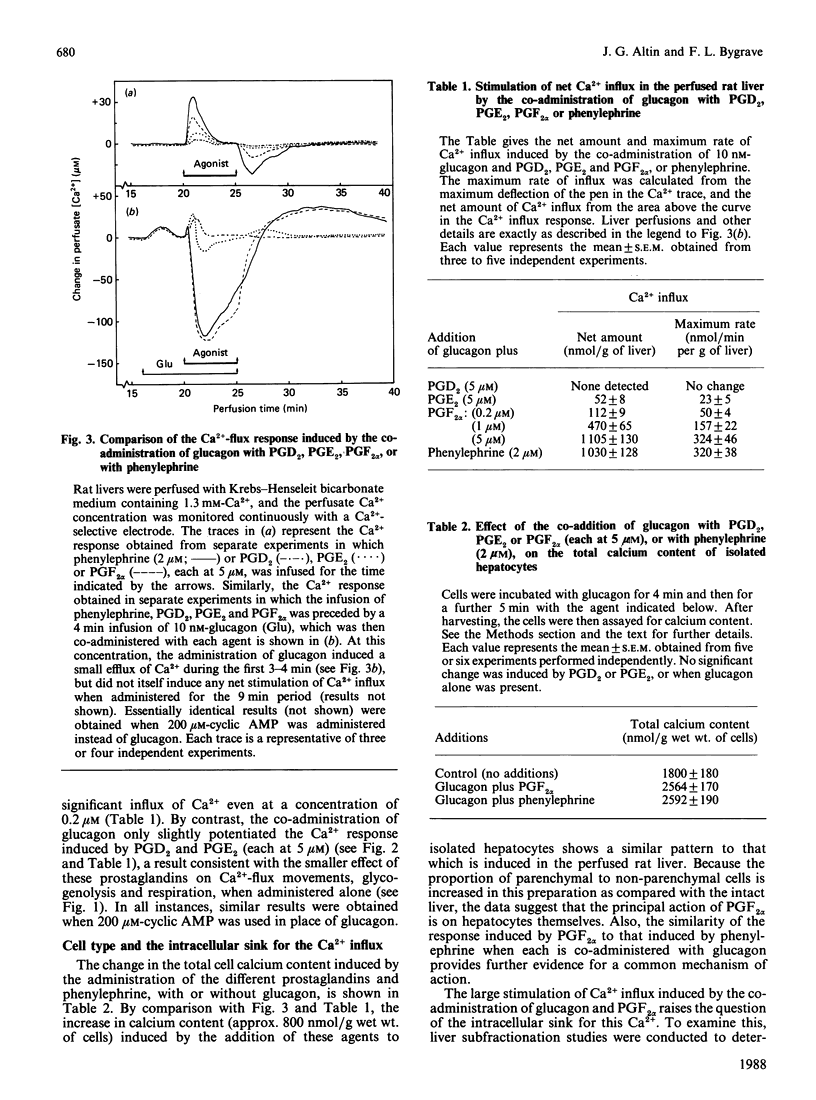
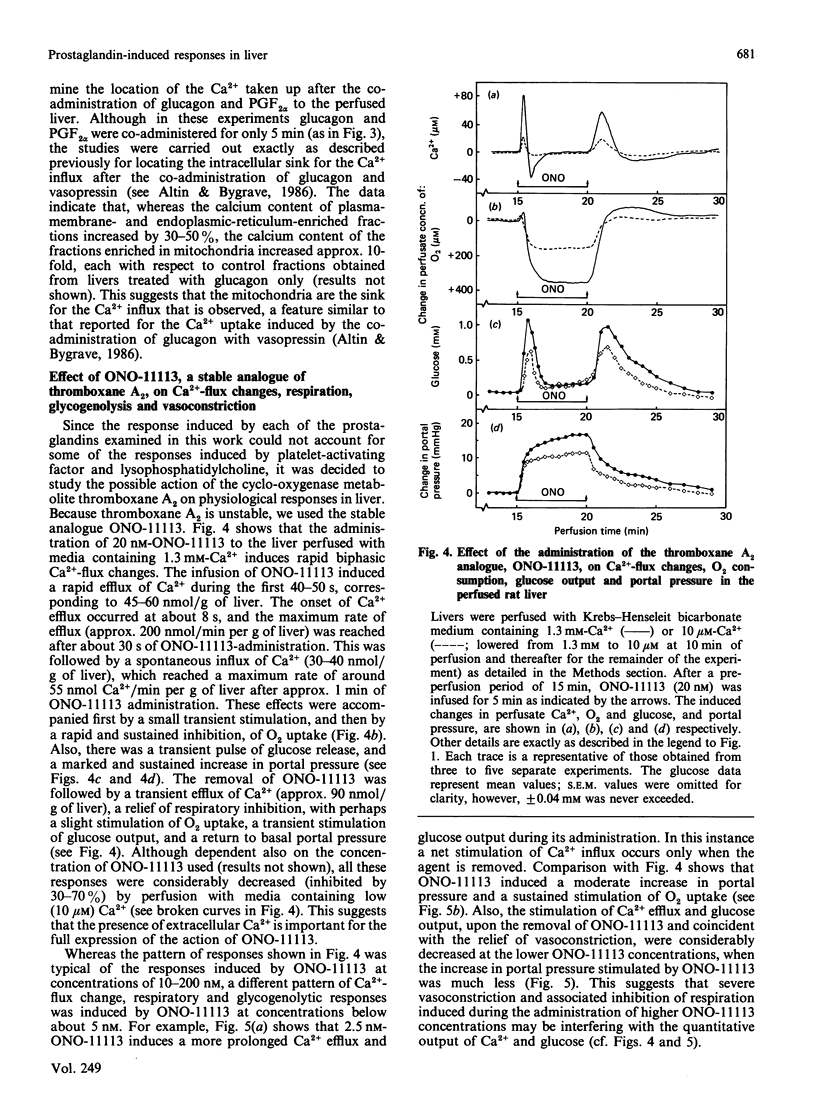
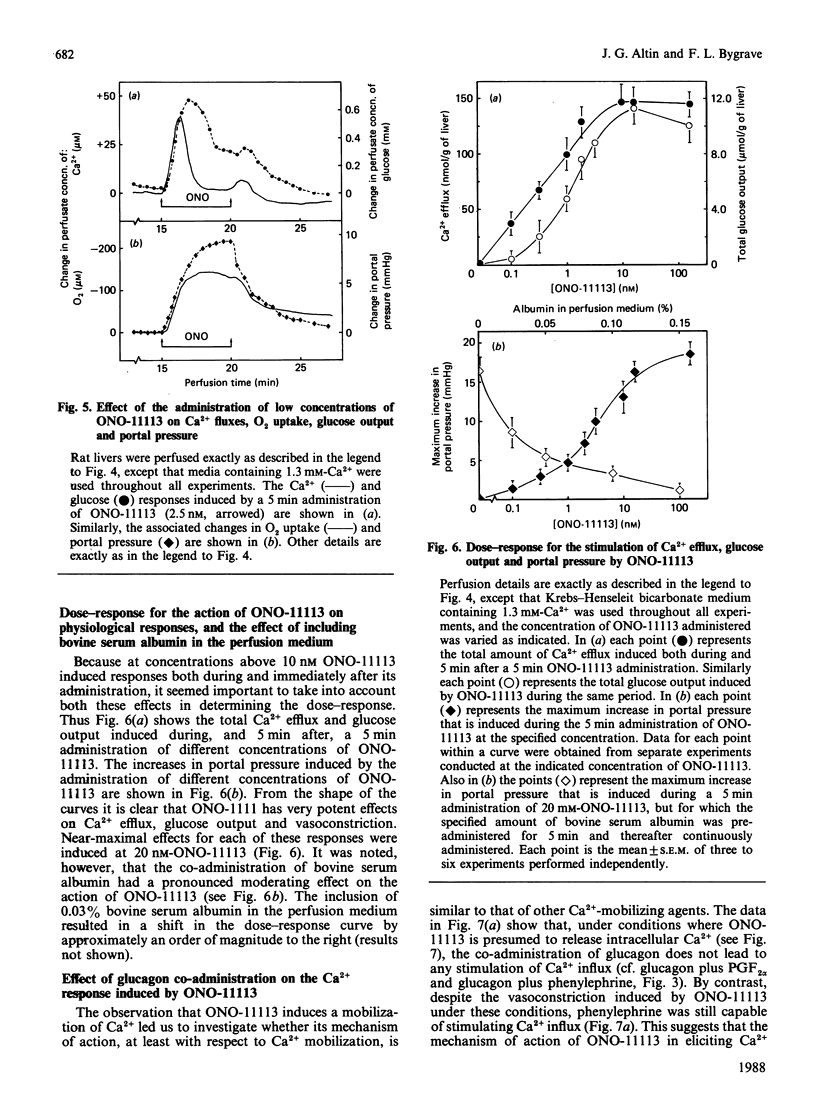
The administration of prostaglandin F2 alpha (PGF2 alpha) and the thromboxane A2 analogue, ONO-11113, to rat livers perfused with media containing either 1.3 mM- or 10 microM-Ca2+ was followed by a stimulation of Ca2+ efflux, changes in O2 uptake and glucose output, and increase in portal pressure. The responses elicited by 5 microM-PGF2 alpha were similar to those induced by the alpha-adrenergic agonist phenylephrine. At both 1.3 mM and 10 microM extracellular Ca2+, PGF2 alpha induced Ca2+ efflux (70-90 nmol/g of liver), probably from the same source as that released by phenylephrine. Prostaglandin D2 (5 microM) and prostaglandin E2 (5 microM) also induced responses, but these were generally much smaller (less than 30%) than those induced by PGF2 alpha. Similarly to vasopressin and other Ca2+-mobilizing hormones, PGF2 alpha also interacted synergistically with glucagon (and cyclic AMP) in stimulating Ca2+ influx both in the perfused liver and in isolated hepatocytes. By comparison with phenylephrine and PGF2 alpha, ONO-11113 was much more potent in inducing vasoconstriction, and, at concentrations of 10-200 nM, induced a different pattern of changes in Ca2+ flux, respiration and glycogenolysis. There was first a rapid efflux of Ca2+ (45-60 nmol/g of liver), followed by a smaller Ca2+ influx, and a further release of Ca2+ (approx. 90 nmol/g of liver) when ONO-11113 was removed. Respiration was first stimulated but then markedly inhibited. At concentrations less than 5 nM, ONO-11113 induced a sustained stimulation of O2 uptake and a more prolonged efflux of Ca2+, with less Ca2+ efflux occurring upon the removal of the agent. Glycogenolysis followed a pattern which was similar to the Ca2+ response. Co-administration of glucagon did not potentiate Ca2+ influx by ONO-11113, but the action of ONO-11113 was inhibited (50%) by a few minutes' prior administration of 10 nM-vasopressin. The vasoconstrictive action of ONO-11113 was synergistically potentiated by the co-administration of phenylephrine. Since the actions of arachidonic acid, platelet-activating factor and lysophosphatidylcholine in liver were recently found to be cyclo-oxygenase-sensitive, the results provide strong evidence that at least PGF2 alpha and thromboxane A2 may be involved in mediating the action of these agents.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altin J. G., Bygrave F. L. Phosphatidic acid and arachidonic acid each interact synergistically with glucagon to stimulate Ca2+ influx in the perfused rat liver. Biochem J. 1987 Nov 1;247(3):613–619. doi: 10.1042/bj2470613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altin J. G., Bygrave F. L. Synergistic stimulation of Ca2+ uptake by glucagon and Ca2+-mobilizing hormones in the perfused rat liver. A role for mitochondria in long-term Ca2+ homoeostasis. Biochem J. 1986 Sep 15;238(3):653–661. doi: 10.1042/bj2380653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altin J. G., Bygrave F. L. The Ca2+-mobilizing actions of vasopressin and angiotensin differ from those of the alpha-adrenergic agonist phenylephrine in the perfused rat liver. Biochem J. 1985 Dec 15;232(3):911–917. doi: 10.1042/bj2320911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altin J. G., Bygrave F. L. The influx of Ca2+ induced by the administration of glucagon and Ca2+-mobilizing agents to the perfused rat liver could involve at least two separate pathways. Biochem J. 1987 Feb 15;242(1):43–50. doi: 10.1042/bj2420043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altin J. G., Dieter P., Bygrave F. L. Evidence that Ca2+ fluxes and respiratory, glycogenolytic and vasoconstrictive effects induced by the action of platelet-activating factor and L-alpha-lysophosphatidylcholine in the perfused rat liver are mediated by products of the cyclo-oxygenase pathway. Biochem J. 1987 Jul 1;245(1):145–150. doi: 10.1042/bj2450145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armato U., Andreis P. G. Prostaglandins of the F series are extremely powerful growth factors for primary neonatal rat hepatocytes. Life Sci. 1983 Oct 31;33(18):1745–1755. doi: 10.1016/0024-3205(83)90681-1. [DOI] [PubMed] [Google Scholar]
- Barritt G. J., Parker J. C., Wadsworth J. C. A kinetic analysis of the effects of adrenaline on calcium distribution in isolated rat liver parenchymal cells. J Physiol. 1981 Mar;312:29–55. doi: 10.1113/jphysiol.1981.sp013614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmelin M., Decker K. Synthesis of prostanoids and cyclic nucleotides by phagocytosing rat Kupffer cells. Eur J Biochem. 1984 Jul 16;142(2):219–225. doi: 10.1111/j.1432-1033.1984.tb08274.x. [DOI] [PubMed] [Google Scholar]
- Brass E. P., Garrity M. J. Effect of E-series prostaglandins on cyclic AMP-dependent and -independent hormone-stimulated glycogenolysis in hepatocytes. Diabetes. 1985 Mar;34(3):291–294. doi: 10.2337/diab.34.3.291. [DOI] [PubMed] [Google Scholar]
- Brass E. P., Garrity M. J., Robertson R. P. Inhibition of glucagon-stimulated hepatic glycogenolysis by E-series prostaglandins. FEBS Lett. 1984 Apr 24;169(2):293–296. doi: 10.1016/0014-5793(84)80336-1. [DOI] [PubMed] [Google Scholar]
- Brønstad G. O., Christoffersen T. Inhibitory effect of prostaglandins on the stimulation by glucagon and adrenaline of formation of cyclic AMP in rat hepatocytes. Eur J Biochem. 1981 Jul;117(2):369–374. doi: 10.1111/j.1432-1033.1981.tb06347.x. [DOI] [PubMed] [Google Scholar]
- Buxton D. B., Hanahan D. J., Olson M. S. Stimulation of glycogenolysis and platelet-activating factor production by heat-aggregated immunoglobulin G in the perfused rat liver. J Biol Chem. 1984 Nov 25;259(22):13758–13761. [PubMed] [Google Scholar]
- Charest R., Prpić V., Exton J. H., Blackmore P. F. Stimulation of inositol trisphosphate formation in hepatocytes by vasopressin, adrenaline and angiotensin II and its relationship to changes in cytosolic free Ca2+. Biochem J. 1985 Apr 1;227(1):79–90. doi: 10.1042/bj2270079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claret-Berthon B., Claret M., Mazet J. L. Fluxes and distribution of calcium in rat liver cells: kinetic analysis and identification of pools. J Physiol. 1977 Nov;272(3):529–552. doi: 10.1113/jphysiol.1977.sp012058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creba J. A., Downes C. P., Hawkins P. T., Brewster G., Michell R. H., Kirk C. J. Rapid breakdown of phosphatidylinositol 4-phosphate and phosphatidylinositol 4,5-bisphosphate in rat hepatocytes stimulated by vasopressin and other Ca2+-mobilizing hormones. Biochem J. 1983 Jun 15;212(3):733–747. doi: 10.1042/bj2120733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J. S., Weakland L. L., Weiland D. A., Farese R. V., West L. A. Prostaglandin F2 alpha stimulates phosphatidylinositol 4,5-bisphosphate hydrolysis and mobilizes intracellular Ca2+ in bovine luteal cells. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3728–3732. doi: 10.1073/pnas.84.11.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRubertis F. R., Zenser T. V., Curnow R. T. Inhibition of glucagon-mediated increases in hepatic cyclic adenosine 3', 5'-monophosphate by prostaglandin E1 and E2. Endocrinology. 1974 Jul;95(1):93–101. doi: 10.1210/endo-95-1-93. [DOI] [PubMed] [Google Scholar]
- Decker K. Eicosanoids, signal molecules of liver cells. Semin Liver Dis. 1985 May;5(2):175–190. doi: 10.1055/s-2008-1063921. [DOI] [PubMed] [Google Scholar]
- Dieter P., Altin J. G., Bygrave F. L. Possible involvement of prostaglandins in vasoconstriction induced by zymosan and arachidonic acid in the perfused rat liver. FEBS Lett. 1987 Mar 9;213(1):174–178. doi: 10.1016/0014-5793(87)81486-2. [DOI] [PubMed] [Google Scholar]
- Dieter P., Altin J. G., Decker K., Bygrave F. L. Possible involvement of eicosanoids in the zymosan and arachidonic-acid-induced oxygen uptake, glycogenolysis and Ca2+ mobilization in the perfused rat liver. Eur J Biochem. 1987 Jun 1;165(2):455–460. doi: 10.1111/j.1432-1033.1987.tb11460.x. [DOI] [PubMed] [Google Scholar]
- Dieter P., Schulze-Specking A., Decker K. Differential inhibition of prostaglandin and superoxide production by dexamethasone in primary cultures of rat Kupffer cells. Eur J Biochem. 1986 Sep 15;159(3):451–457. doi: 10.1111/j.1432-1033.1986.tb09907.x. [DOI] [PubMed] [Google Scholar]
- Exton J. H. Mechanisms involved in alpha-adrenergic phenomena. Am J Physiol. 1985 Jun;248(6 Pt 1):E633–E647. doi: 10.1152/ajpendo.1985.248.6.E633. [DOI] [PubMed] [Google Scholar]
- Fisher R. A., Robertson S. M., Olson M. S. Stimulation of glycogenolysis and vasoconstriction in the perfused rat liver by the thromboxane A2 analogue U-46619. J Biol Chem. 1987 Apr 5;262(10):4631–4638. [PubMed] [Google Scholar]
- García-Sáinz J. A., Hernández-Sotomayor S. M. Stimulation of hepatic glycogenolysis by 12-O-tetradecanoyl-phorbol-13-acetate (TPA) via cyclooxygenase products. Biochem Biophys Res Commun. 1985 Oct 15;132(1):204–209. doi: 10.1016/0006-291x(85)91008-3. [DOI] [PubMed] [Google Scholar]
- Hems D. A., Whitton P. D. Control of hepatic glycogenolysis. Physiol Rev. 1980 Jan;60(1):1–50. doi: 10.1152/physrev.1980.60.1.1. [DOI] [PubMed] [Google Scholar]
- Hers H. G. The control of glycogen metabolism in the liver. Annu Rev Biochem. 1976;45:167–189. doi: 10.1146/annurev.bi.45.070176.001123. [DOI] [PubMed] [Google Scholar]
- Joseph S. K., Thomas A. P., Williams R. J., Irvine R. F., Williamson J. R. myo-Inositol 1,4,5-trisphosphate. A second messenger for the hormonal mobilization of intracellular Ca2+ in liver. J Biol Chem. 1984 Mar 10;259(5):3077–3081. [PubMed] [Google Scholar]
- Macphee C. H., Drummond A. H., Otto A. M., Jimenez de Asua L. Prostaglandin F2 alpha stimulates phosphatidylinositol turnover and increases the cellular content of 1,2-diacylglycerol in confluent resting Swiss 3T3 cells. J Cell Physiol. 1984 Apr;119(1):35–40. doi: 10.1002/jcp.1041190107. [DOI] [PubMed] [Google Scholar]
- Mauger J. P., Poggioli J., Claret M. Synergistic stimulation of the Ca2+ influx in rat hepatocytes by glucagon and the Ca2+-linked hormones vasopressin and angiotensin II. J Biol Chem. 1985 Sep 25;260(21):11635–11642. [PubMed] [Google Scholar]
- Mendlovic F., Corvera S., García-Sáinz J. A. Possible involvement of cyclooxygenase products in the actions of platelet-activating factor and of lipoxygenase products in the vascular effects of epinephrine in perfused rat liver. Biochem Biophys Res Commun. 1984 Sep 17;123(2):507–514. doi: 10.1016/0006-291x(84)90259-6. [DOI] [PubMed] [Google Scholar]
- Mene P., Dubyak G. R., Scarpa A., Dunn M. J. Stimulation of cytosolic free calcium and inositol phosphates by prostaglandins in cultured rat mesangial cells. Biochem Biophys Res Commun. 1987 Jan 30;142(2):579–586. doi: 10.1016/0006-291x(87)90313-5. [DOI] [PubMed] [Google Scholar]
- Morgan N. G., Blackmore P. F., Exton J. H. Modulation of the alpha 1-adrenergic control of hepatocyte calcium redistribution by increases in cyclic AMP. J Biol Chem. 1983 Apr 25;258(8):5110–5116. [PubMed] [Google Scholar]
- Okamura N., Terayama H. Prostaglandin receptor-adenylate cyclase system in plasma membranes of rat liver and ascites hepatomas, and the effect of GTP upon it. Biochim Biophys Acta. 1977 Feb 14;465(1):54–67. doi: 10.1016/0005-2736(77)90355-8. [DOI] [PubMed] [Google Scholar]
- Okumura T., Sago T., Saito K. Prostaglandin E1 binding protein on the surface of primary cultured hepatocytes. Biochem Int. 1987 Mar;14(3):443–449. [PubMed] [Google Scholar]
- Poggioli J., Mauger J. P., Claret M. Effect of cyclic AMP-dependent hormones and Ca2+-mobilizing hormones on the Ca2+ influx and polyphosphoinositide metabolism in isolated rat hepatocytes. Biochem J. 1986 May 1;235(3):663–669. doi: 10.1042/bj2350663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhart P. H., Taylor W. M., Bygrave F. L. Calcium ion fluxes induced by the action of alpha-adrenergic agonists in perfused rat liver. Biochem J. 1982 Dec 15;208(3):619–630. doi: 10.1042/bj2080619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhart P. H., Taylor W. M., Bygrave F. L. The role of calcium ions in the mechanism of action of alpha-adrenergic agonists in rat liver. Biochem J. 1984 Oct 1;223(1):1–13. doi: 10.1042/bj2230001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smigel M., Fleischer S. Characterization of triton X-100-solubilized prostaglandin E binding protein of rat liver plasma membranes. J Biol Chem. 1977 Jun 10;252(11):3689–3696. [PubMed] [Google Scholar]
- Williamson J. R., Cooper R. H., Joseph S. K., Thomas A. P. Inositol trisphosphate and diacylglycerol as intracellular second messengers in liver. Am J Physiol. 1985 Mar;248(3 Pt 1):C203–C216. doi: 10.1152/ajpcell.1985.248.3.C203. [DOI] [PubMed] [Google Scholar]


