FIGURE 4.
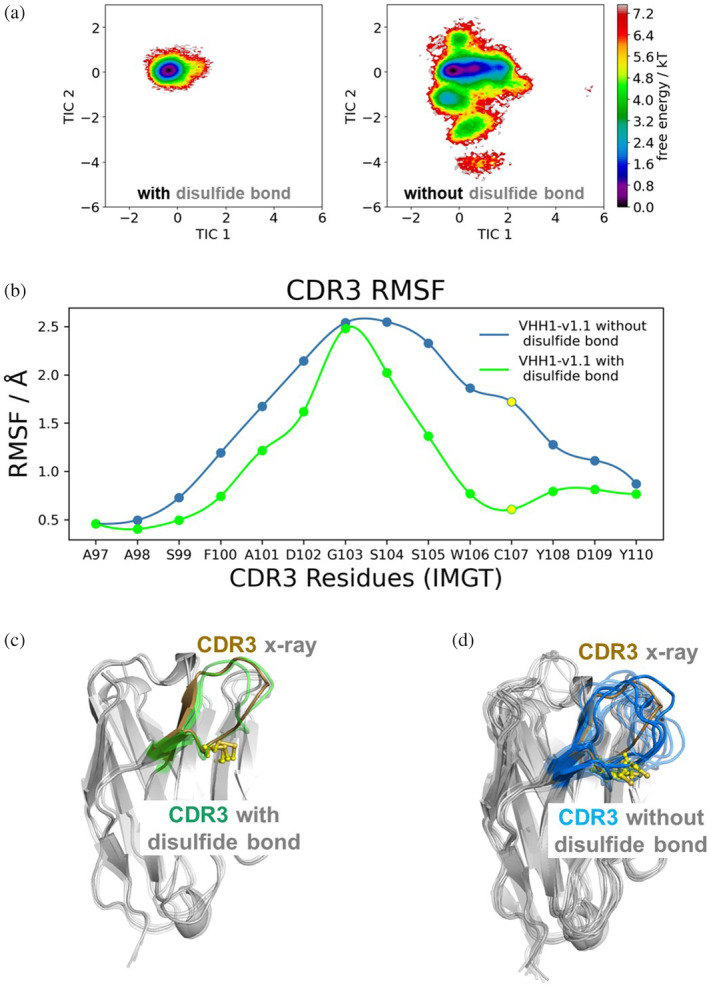
Non‐canonical disulfide bridge substantially stabilizes the CDR3 loop in the binding competent conformation. (a) Free energy landscape of the CDR3 loop for VHH1‐v1.0 with and without disulfide bridge shows a substantial increase in conformational diversity. (b) Root mean square fluctuation (RMSF) for CDR3 residues for the MD simulations of VHH1‐v1.1 with and without the disulfide bridge between residues Cys55 and Cys107 (highlighted in yellow). Structural visualization of the conformational ensembles obtained from the clustering analysis using the same RMSD distance cut‐off criterion (2.5 Å) for VHH1‐v1.1 (c) with and (d) without disulfide bond between non‐canonical cysteines Cys55 and Cys107, aligned to the x‐ray structure of VHH1‐v1.0 (PDB code 9FXF), illustrating that the formation of the cysteine bridge stabilizes the binding competent CDR3 conformation.
