FIGURE 6.
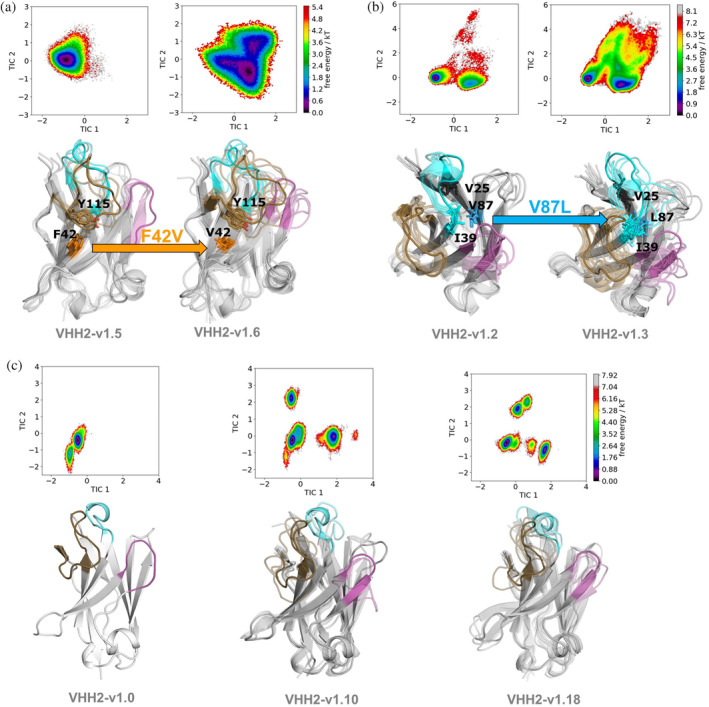
Structural and dynamic characterization of Hallmark mutations suggesting conformational entropy as critical determinant for antigen‐recognition. (a) Mutation F42V destabilizes the CDR3 loop, resulting in a bigger conformational space and a higher number of low populated states. The higher conformational diversity of the CDR3 loop is also reflected in the broader conformational ensemble. (b) In addition to directly stabilizing the CDR loops, the Hallmark residue mutation V87L reveals a conformational rearrangement of the CDR2 and CDR1 loops to accommodate the bulkier leucine sidechain. (c, d) Free energy surface of the paratope for VHH2‐v1.0 compared with VHH2‐v1.10 and VHH2‐v1.18. Both VHH2‐v1.10 and VHH2‐v1.18 are non‐binders and reveal a substantially increased conformational diversity reflected in a broader conformational ensemble, accompanied by a substantial population shift towards three equally lower populated states in contrast to one dominant state corresponding to the binding competent state for VHH2‐v1.0.
