Abstract
Background
Bicuspid aortic valve (BAV) is often associated with a concomitant aortopathy. However, few studies have evaluated the effect of the aortic valve (AV) phenotype on the rate of dilation of the aorta. This study aimed to compare the progression rate of aorta dimensions according to AV phenotype (BAV vs tricuspid AV (TAV)), fusion type and sex in patients with aortic stenosis (AS).
Methods
310 patients with AS (224 TAV and 86 BAV) recruited in the Metabolic Determinants of the Progression of Aortic Stenosis study (PROGRESSA, NCT01679431) were included in this analysis. Doppler echocardiography was performed annually to assess AS severity and measure ascending aorta (AA) dimensions. Baseline and last follow-up visit measurements were used to assess the annualised change.
Results
Median AA annualised change was larger in BAV versus TAV (0.33±0.65 mm/year vs 0.21±0.56 mm/year, p=0.04). In the whole cohort, BAV phenotype and higher low-density lipoprotein (LDL) levels were significantly associated with fast progression of AA dilation in univariate analysis (OR 1.80, 95% CI 1.08 to 2.98, p=0.02; 1.37, 95% CI 1.04 to 1.80, p=0.03, respectively). AA dilation rate did not vary according to the BAV subtype (p=0.142). Predictors of AA progression rate were different between valve phenotypes, with higher apolipoprotein B/apolipoprotein A-I ratio, higher baseline peak aortic jet velocity (Vpeak) and smaller baseline AA diameter in the TAV cohort (all p<0.05) versus absence of hypertension, higher LDL levels and smaller baseline AA diameter in the BAV cohort (all p<0.02). In men, higher baseline Vpeak and smaller baseline AA (p<0.001) were independently associated with increased annualised AA dilation, while in women, higher LDL levels (p=0.026) were independently associated with faster AA dilation.
Conclusion
This study suggests that BAV is associated with faster dilation of the AA. Predictors of AA dilation are different between valve phenotype and sex, with higher LDL levels being associated with faster AA dilation in BAV.
Keywords: Congenital Abnormalities, Aortic Diseases, Echocardiography, Heart Valve Diseases
WHAT IS ALREADY KNOWN ON THIS TOPIC
Some previous studies reported a faster dilation rate in patients with bicuspid aortic valve (BAV), while others reported no impact of valve phenotype on ascending aorta (AA) dilation rate.
WHAT THIS STUDY ADDS
BAV is associated with faster dilation of the AA compared to tricuspid aortic valve (TAV). Predictors of AA dilation are different between valve phenotype and sex, with higher LDL levels being associated with faster AA dilation in BAV.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Closer clinical and imaging follow-up should be considered in patients with BAV dilation. Aggressive management of dyslipidemia may contribute to prevent or slow AA dilation in patients with BAV.
Introduction
Bicuspid aortic valve (BAV) is the most common form of congenital heart disease, with a prevalence of approximately 1%–2% in the general population.1 This congenital abnormality is associated with a high risk of developing aortic valve dysfunction (stenosis or regurgitation)2 and/or aortopathy.3 4 For these reasons, patients with BAV represent approximately 50% of those undergoing aortic valve replacement (AVR).5 6 BAV-related aortopathy is associated with a sixfold to ninefold increased risk of aorta complications, such as aortic rupture or dissection.7 Genetic, haemodynamic and structural factors have been suggested to explain the higher prevalence of aorta dilation in patients with BAV.8 9 However, the data on the impact of valve phenotype on the progression rate of ascending aorta (AA) dilation progression remain conflicting, with some studies reporting an influence of valve morphology on AA dilation rate,10 with a faster dilation rate in patients with BAV, while others reporting no impact of valve phenotype on AA dilation rate.11 It is important to note that these studies included a low number of patients with BAV. Current guidelines for the treatment of AA dilation are specifically tailored to patients with BAV.12 13
The aims of the present study were to (1) evaluate the impact of aortic valve phenotype on the rate of AA and aortic root (AR) dilation; (2) determine which factors are associated with faster AA dilation in BAV and in patients with tricuspid aortic valve (TAV) and (3) assess the effect of sex and BAV fusion type on dilation progression rates.
Methods
Study population
310 patients with at least mild aortic stenosis (AS) recruited in the prospective observational Metabolic Determinants of the Progression of Aortic Stenosis (PROGRESSA) study (Clinical trial register: NCT01679431) between 2005 and 2022 were included in this subanalysis. The design of the PROGRESSA study has been described in detail.14,16 The study population was divided according to aortic valve phenotype (patients with TAV: n=224; patients with BAV: n=86). Demographic, clinical and Doppler echocardiographic data were prospectively gathered yearly. Exclusion criteria were symptomatic AS, moderate to severe aortic regurgitation, significant mitral valve disease, rheumatic valvular disease or endocarditis, reduced ejection fraction (<50%), previous aortic or mitral valve repair/replacement, previous AA repair/replacement and pregnancy. Plasma levels of glucose, creatinine, N-terminal pro-b-type natriuretic peptide (Nt-pro-BNP), high-sensitivity troponin T, standard lipid profile, apolipoprotein B (apo B), apolipoprotein A-I (apo A-I) and standard haematology profile were measured from fasting blood samples using automated techniques standardised by the Canadian reference laboratory. This study was approved by the Quebec Heart and Lung Institute Ethics Committee, and all patients signed a written informed consent at the time of inclusion.
Doppler echocardiography data
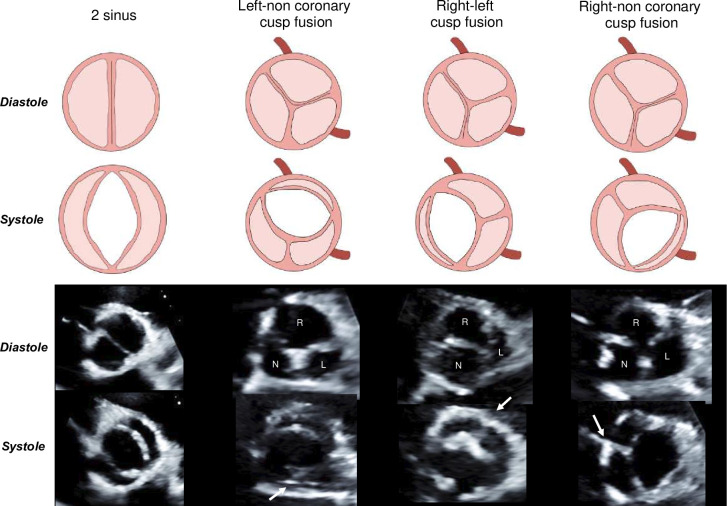
Transthoracic echocardiographic examinations were performed using commercially available ultrasound systems at baseline and yearly following enrolment by experienced sonographers. Aortic valve morphology was assessed on a short-axis view according to the system proposed by Michelena et al.17 (figure 1). The left ventricular outflow tract (LVOT) diameter was measured at the insertion of the aortic valve leaflets in a parasternal long-axis zoom view. Stroke volume (SV) was calculated by multiplying the LVOT area by the velocity time integral obtained by pulsed wave Doppler in the LVOT. SV was then indexed by body surface area (BSA) to obtain the SV index. Haemodynamic parameters used to evaluate AS severity were peak aortic jet velocity (Vpeak) measured by continuous wave Doppler, mean transvalvular gradient (MG) derived from the modified Bernoulli equation and aortic valve area (AVA) calculated by the standard continuity equation. AA and AR dimensions were measured in parasternal long axis view from leading edge to leading edge, in a perpendicular fashion to the aorta in end-diastole, according to the American Society of Echocardiography and the European Association of Cardiovascular Imaging recommendations.18 19 AR diameter was measured at the sinus of Valsalva. Left ventricular ejection fraction (LVEF) was measured using the Simpson biplane method. Global left ventricular afterload was estimated using valvulo-arterial impedance calculated according to Briand et al.20 The energy loss index was calculated according to the method proposed by Garcia et al.21
Figure 1. Bicuspid leaflet fusion subtypes. Determination of bicuspid aortic valve leaflet fusion subtypes using parasternal short axis echocardiographic view in systole and diastole based on the Michelena et al. classification. The white arrow indicates the raphe. N, non-coronary leaflet; L, left coronary leaflet; R, right coronary leaflet.
Left ventricular (LV) mass was calculated by the modified American Society of Echocardiography formula and subsequently indexed to BSA.18 To accommodate for the various follow-up times, aorta dilation was annualised to better compare dilation rates between patients with different follow-up durations. Annualised AA dilation rate was defined as (AA diameter at last follow-up–AA diameter at baseline)/follow-up time. The same method was used to evaluate AR annualised progression. Fast progression of AA dilation was defined as a value above the median annualised diameter variation (0.18 mm/year) in the whole cohort. Finally, other echocardiographic measurements were performed according to the European Association of Cardiovascular Imaging and American Society of Echocardiography guidelines.18
Statistical analyses
Continuous variables were tested for normality by the Shapiro-Wilk or the Kolmogorov-Smirnov tests and presented as mean±SD. According to normal or non-parametric distributions, a Student’s t-test or a Mann-Whitney test was performed to evaluate differences between groups. Categorical variables were expressed as a number of patients (per cent) and compared using the χ2 or Fischer’s exact test. Univariate and multivariate linear and logistic regression analyses were performed to determine factors associated with both AA and AR dilation individually. The AA dilation rate was used in these models in two forms: as a continuous variable and as a dichotomous variable to separate patients with fast progression and slow progression. Linear mixed-effects models were used to show and compare AA and AR diameters over time according to valve phenotype.22 A composite endpoint including all-cause mortality and AVR was used to assess the association with AA dilation using the Cox proportional hazards regression analysis. Statistical significance was defined as p<0.05. Statistical analyses were performed using SPSS V.29.0 (IBM Corporation, Armonk, New York, USA) and STATA V.17.0 (StataCorp, College Station, Texas, USA).
Results
Baseline clinical and echocardiographic characteristics
Baseline patient characteristics according to valve phenotype are presented in table 1. Among the 310 patients included in this study, 224 (72%) had TAV and 86 (28%) had BAV. As expected, patients with BAV were significantly younger, had a lower body mass index (BMI) and had significantly less hypertension, diabetes and coronary artery disease than patients with TAV (all p<0.001). Patients with BAV also presented lower fasting glucose, creatinine, NT-pro-BNP, high-sensitivity troponin and triglycerides (all p<0.001). However, patients with BAV had significantly higher low-density lipoprotein (LDL) levels (p<0.001).
Table 1. Patient characteristics according to valve phenotype.
| Whole cohort(n=310) | TAV(n=224, 72%) | BAV(n=86, 28%) | P value | |
| Clinical data | ||||
| Age, years | 63±14 | 70±8 | 48±14 | <0.001 |
| Male sex | 222 (72) | 172 (77) | 50 (58) | 0.001 |
| Body mass index, kg/m2 | 28.4±4.4 | 28.9±4.4 | 27.0±4.2 | <0.001 |
| Hypertension | 2 (77) | 198 (88) | 40 (47) | <0.001 |
| Metabolic syndrome | 78 (25) | 69 (31) | 9 (10) | <0.001 |
| Diabetes | 75 (24) | 78 (30) | 7 (8) | <0.001 |
| Coronary artery disease | 202 (65) | 174 (78) | 28 (33) | <0.001 |
| Hypertension medication | 215 (69) | 182 (81) | 33 (38) | <0.001 |
| Hypolipidemic medication | 199 (64) | 170 (76) | 29 (34) | <0.001 |
| Previous coarctation repair | 4 (1) | 0 (0) | 4 (5) | <0.001 |
| Metabolic data | ||||
| Fasting glucose, mmol/L | 5.8±1.5 | 6.0±1.6 | 5.3±1.0 | <0.001 |
| Low-density lipoprotein, mmol/L | 2.33±0.84 | 2.20±0.80 | 2.65±0.85 | <0.001 |
| High-density lipoprotein, mmol/L | 1.45±0.40 | 1.44±0.41 | 1.49±0.38 | 0.111 |
| Triglycerides, mmol/L | 1.41±0.75 | 1.41±0.65 | 1.39±0.97 | 0.032 |
| Apolipoprotein/apolipoprotein A1-I ratio | 0.57±0.18 | 0.57±0.17 | 0.59±0.21 | 0.652 |
| Creatinine, µmol/L | 84±24 | 87±26 | 76±16 | <0.001 |
| N-terminal pro b-type natriuretic peptide, ng/L | 164±223 | 201±237 | 68±145 | <0.001 |
| High sensitivity troponin, ng/L | 10±7 | 11±7 | 5±3 | <0.001 |
| Echocardiographic data | ||||
| Left ventricular outflow tract diameter, mm | 22.3±2.2 | 21.8±1.9 | 23.4±2.6 | <0.001 |
| Peak aortic jet velocity, cm/s | 273±51 | 275±50 | 269±54 | 0.191 |
| Mean gradient, mm Hg | 17.7±7.9 | 17.6±7.8 | 17.9±8.2 | 0.935 |
| Aortic valve area, cm2 | 1.27±0.31 | 1.24±0.29 | 1.34±0.36 | 0.034 |
| Indexed aortic valve area, cm2/m2 | 0.67±0.16 | 0.66±0.15 | 0.72±0.17 | 0.004 |
| Stroke volume, mL | 79±15 | 78±14 | 81±17 | 0.082 |
| Stroke volume index, mL/m2 | 42±7 | 41±7 | 43±8 | 0.113 |
| Left ventricular mass, g | 198±51 | 201±48 | 188±59 | 0.042 |
| Left ventricular ejection fraction, % | 64±6 | 65±6 | 64±5 | 0.128 |
| Relative wall thickness ratio | 0.48±0.09 | 0.50±0.08 | 0.43±0.08 | <0.001 |
| Valvulo-arterial impedance, mm Hg/mL/m2 | 3.77±0.73 | 3.90±0.71 | 3.44±0.68 | <0.001 |
| Energy loss index, cm2/m2 | 1.13±0.61 | 1.10±0.58 | 1.21±0.66 | 0.112 |
| Baseline aortic root diameter, mm | 34.1±4.4 | 34.0±4.1 | 34.2±4.9 | 0.936 |
| Baseline ascending aorta diameter, mm | 35.5±5.1 | 34.5±4.3 | 37.9±6.1 | <0.001 |
| Annualised aortic root dilation, mm/year | 0.25±0.82 | 0.25±0.89 | 0.27±0.60 | 0.625 |
| Annualised ascending aorta dilation, mm/year | 0.25±0.60 | 0.21±0.56 | 0.33±0.65 | 0.043 |
Continuous data are expressed mean±SD. Categorical data are expressed by number (per cent). P values refer to comparison between BAV and TAV groups. Values in bold reach statistical significance (p<0.05).
BAVbicuspid aortic valveTAVtricuspid aortic valve
Baseline echocardiographic data according to valve phenotype are presented in table 1. There was no significant difference between BAV and TAV concerning Vpeak, SV index and MG. Patients with BAV had lower LV mass, relative wall thickness ratio and valvulo-arterial impedance while having significantly larger AVA, indexed AVAi, baseline AA and LVOT diameters (all p<0.05).
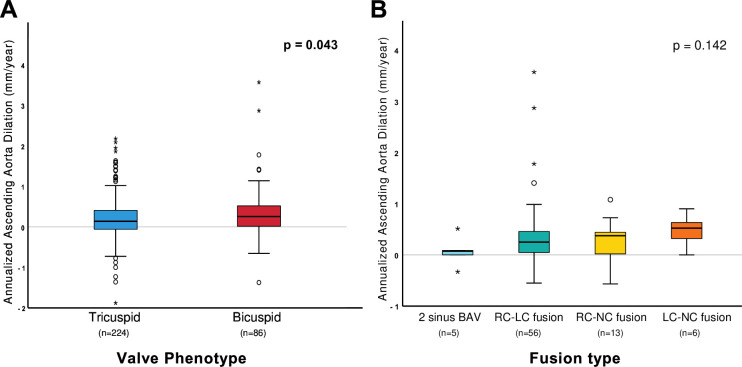
Annualised progression rate of AA dilation was significantly higher in patients with BAV compared with patients with TAV (0.21±0.56 vs 0.33±0.65 mm/year, respectively, p=0.043; figure 2 panel A). This association was confirmed using linear mixed-effect models with significantly faster dilation of AA in patients with BAV at 3 and 5 years of follow-up (figure 3 panel A). Separate linear mixed-effect models were performed according to sex (figure 3 panels B and C). There was no significant difference between patients with TAV and BAV in regard to AR dilation rate (p=0.625) (online supplemental figure 1). Linear mixed-effect models of AR dilation according to sex can be observed in online supplemental figure 2. The progression rate of AA and AR dilation according to valve phenotype and sex is presented in online supplemental figure 3.
Figure 2. Annualised AA dilation rate according to valve phenotype and fusion type. Box-plot representation of annualised AA dilation rates. Panel A compares the AA dilation rate between bicuspid and tricuspid patients. Panel B compares AA dilation rates according to the BAV subtype. AA, ascending aorta; BAV, bicuspid aortic valve; RC-LC, right-left coronary fusion; RC-NC, right-non coronary fusion; LC-NC, left-non coronary fusion.
Figure 3. Linear prediction models of AA size according to aortic valve phenotype and sex. Evolution of AA diameter during follow-up according to valve phenotype using linear mixed models. Panel A demonstrates statistically faster AA dilation in patients with BAV at 4 and 5 years of follow-up. Panel B is a subanalysis including only male patients showing no statistical differences. Panel C is a subanalysis that includes only female patients showing faster AA dilation in BAV women at 4 and 5 years. AA, ascending aorta; BAV, bicuspid aortic valve; TAV, tricuspid aortic valve.

Predictors of AA dilation
Whole cohort
Univariate and multivariate linear regression models were performed using AA dilation as a continuous variable (table 2). In univariate analysis, younger age (standardised beta=−0.15±0.01, p=0.007), absence of hypertension (standardised beta=−0.19±0.08, p<0.001), LDL levels (standardised beta=0.18±0.04, p=0.001), Apo B/Apo A-I ratio (standardised beta=0.12±0.18, p=0.042) and smaller baseline AA diameter (standardised beta=−0.17±0.01, p=0.003) were associated with increased AA dilation rate. In a multivariate analysis, including the former variables, only a smaller baseline AA diameter remained significantly associated with AA dilation (standardised beta=−0.19±0.01, p<0.001).
Table 2. Univariate and multivariate linear regression models of ascending aorta dilation as a continuous variable for the whole cohort.
| Univariate | Multivariate | |||
| Standardised beta±SE | P value | Standardisedbeta±SE | P value | |
| Age, years | −0.15±0.01 | 0.007 | −0.09±0.01 | 0.165 |
| Sex | −0.03±0.07 | 0.567 | ||
| Bicuspid aortic valve | 0.09±0.08 | 0.118 | ||
| Hypertension | −0.19±0.08 | <0.001 | −0.11±0.09 | 0.085 |
| History of smoking | −0.01±0.07 | 0.841 | ||
| Diabetes | −0.10±0.08 | 0.066 | ||
| Glycaemia, mmol/L | −0.11±0.02 | 0.058 | ||
| Body mass index, kg/m2 | −0.06±0.01 | 0.297 | ||
| Body surface area, m2 | 0.01±0.16 | 0.860 | ||
| Low-density lipoprotein, mmol/L | 0.18±0.04 | 0.001 | 0.10±0.05 | 0.158 |
| Apolipoprotein B/apolipoprotein A1-I ratio | 0.12±0.18 | 0.042 | 0.06±0.22 | 0.351 |
| High-density lipoprotein, mmol/L | −0.07±0.09 | 0.209 | ||
| Triglycerides, mmol/L | 0.09±0.05 | 0.139 | ||
| Systolic blood pressure, mm Hg | −0.09±0.01 | 0.124 | ||
| Diastolic blood pressure, mm Hg | −0.01±0.01 | 0.800 | ||
| Valvulo-arterial impedance, mm Hg/mL/m2 | −0.01±0.05 | 0.954 | ||
| Vpeak baseline, cm/s | 0.11±0.01 | 0.062 | ||
| ∆Vpeak, cm/s | 0.06±0.01 | 0.328 | ||
| Ascending aorta diameter, mm | −0.17±0.01 | 0.003 | −0.19±0.01 | <0.001 |
Values in bold reach statistical significance (p<0.05).
Vpeakpeak aortic jet velocity
The cohort was also divided into two groups based on AA dilation rate, categorised as slow progressors (<0.18 mm/year) or fast progressors (≥0.18 mm/year). In the whole cohort, 52 (60%) of the patients with BAV and 103 (46%) of the patients with TAV had a fast progression of the AA dilation (p=0.03). The factors associated with the fast progression of AA dilation in univariate analysis are presented in online supplemental table 1. In the whole cohort, BAV phenotype (OR 1.80, 95% CI 1.08 to 2.98, p=0.02), younger age (OR 0.98, 95% CI 0.96 to 0.99, p<0.01) and higher LDL levels (OR 1.37, 95% CI 1.04 to 1.80, p=0.03) were significantly associated with fast progression of AA dilation. No factors remained significantly associated with fast AA dilation in a multivariate model (all p>0.08).
Sex-specific analysis
Linear multivariate regression models were performed for both men and women from the whole cohort to identify the factors associated with a faster progression rate of AA dilation as a continuous variable (table 3). Multivariate linear regression was adjusted for age, BAV, hypertension, glycaemia, LDL levels, baseline Vpeak and baseline AA diameter. In men, higher baseline Vpeak (p=0.015) and smaller baseline AA (p<0.001) were independently associated with increased annualised AA dilation. For women, only higher LDL levels (p=0.026) were independently associated with faster AA dilation. Logistic univariate models according to sex can be seen in online supplemental table 2. Significant factors associated with fast AA dilation in women were BAV (OR 3.47, 95% CI 1.42 to 8.49, p=0.006), younger age (OR 0.96, 95% CI 0.93 to 0.99, p=0.004) and LDL levels (OR 1.71, 95% CI 1.02 to 2.86, p=0.043). No significant factors were specifically identified for men.
Table 3. Linear multivariate regression models of ascending aorta dilation as a continuous variable according to sex.
| Men | Women | |||
| Standardised beta±SE | P value | Standardised beta±SE | P value | |
| Age, years | −0.13±0.01 | 0.197 | −0.09±0.01 | 0.582 |
| Bicuspid aortic valve | −0.02±0.14 | 0.877 | 0.15±0.19 | 0.348 |
| Hypertension | −0.09±0.11 | 0.211 | −0.11±0.15 | 0.380 |
| Glycaemia, mmol/L | −0.07±0.03 | 0.300 | −0.10±0.05 | 0.356 |
| Low-density lipoprotein, mmol/L | 0.09±0.05 | 0.209 | 0.24±0.07 | 0.026 |
| Peak aortic jet velocity, cm/s | 0.16±0.01 | 0.015 | −0.07±0.01 | 0.518 |
| Ascending aorta diameter, mm | −0.28±0.01 | <0.001 | −0.09±0.02 | 0.462 |
Values in bold reach statistical significance (p<0.05).
Valve phenotype analysis
The factors associated with the progression of AA dilation as a continuous variable according to valve phenotype are presented in table 4. In patients with TAV, higher ApoB/ApoA-I ratio and higher baseline Vpeak were associated with faster AA dilation (standardised beta=0.14±0.22, p=0.041; beta=0.16±0.01, p=0.018, respectively). In patients with BAV, higher LDL levels were associated with faster AA dilation (standardised beta=0.25±0.08, p=0.016) and hypertension was associated with slower AA dilation (standardised beta=−0.27±0.14, p=0.013). Smaller baseline AA diameter was associated with faster AA dilation in both patients with TAV and BAV (standardised beta=−0.14±0.01, p=0.027; beta=−0.31±0.01, p=0.003, respectively). Factors associated with fast AA dilation according to valve phenotype are presented in online supplemental table 3.
Table 4. Linear regression models of ascending aorta dilation as a continuous variable according to aortic valve phenotype.
| TAV(n=224) | BAV(n=86) | |||||||
| Univariate | Multivariate | Univariate | Multivariate | |||||
| Standardisedbeta±SE | Pvalue | Standardisedbeta±SE | Pvalue | Standardisedbeta±SE | Pvalue | Standardisedbeta±SE | Pvalue | |
| Age | −0.11±0.01 | 0.099 | −0.16±0.01 | 0.161 | ||||
| Sex | −0.12±0.09 | 0.073 | 0.08±0.14 | 0.441 | ||||
| Hypertension | −0.10±0.12 | 0.140 | −0.27±0.14 | 0.013 | −0.18±0.14 | 0.085 | ||
| History of smoking | −0.08±0.08 | 0.266 | 0.04±0.15 | 0.711 | ||||
| Diabetes | 0.09±0.08 | 0.178 | 0.09±0.26 | 0.435 | ||||
| Glycaemia, mmol/L | −0.10±0.02 | 0.121 | −0.07±0.07 | 0.536 | ||||
| Body mass index, kg/m2 | −0.08±0.01 | 0.265 | 0.03±0.02 | 0.744 | ||||
| Body surface area, m2 | 0.00±0.20 | 0.994 | 0.04±0.33 | 0.705 | ||||
| Low-density lipoprotein, mmol/L | 0.12±0.05 | 0.066 | 0.26±0.08 | 0.016 | 0.25±0.08 | 0.016 | ||
| Apolipoprotein B/apolipoprotein A1-I | 0.14±0.22 | 0.041 | 0.14±0.22 | 0.031 | 0.07±0.33 | 0.543 | ||
| High-density lipoprotein, mmol/L | −0.05±0.09 | 0.456 | −0.15±0.19 | 0.179 | ||||
| Triglycerides, mmol/L | 0.02±0.06 | 0.725 | 0.18±0.07 | 0.096 | ||||
| Systolic blood pressure, mm Hg | −0.05±0.01 | 0.358 | −0.10±0.01 | 0.373 | ||||
| Diastolic blood pressure, mm Hg | 0.05±0.01 | 0.502 | −0.21±0.01 | 0.057 | ||||
| Valvulo-arterial impedance, mm Hg/mL/m2 | 0.06±0.06 | 0.411 | −0.04±0.10 | 0.737 | ||||
| Vpeak, cm/s | 0.16±0.01 | 0.018 | 0.16±0.01 | 0.015 | 0.01±0.01 | 0.898 | ||
| ∆Vpeak, cm/s | 0.02±0.01 | 0.710 | 0.16±0.01 | 0.164 | ||||
| Ascending aorta diameter, mm | −0.14±0.01 | 0.038 | −0.15±0.01 | 0.027 | −0.31±0.01 | 0.003 | −0.26±0.01 | 0.014 |
Values in bold reach statistical significance (p<0.05).
∆Vpeakannualised peak aortic jet velocity progressionVpeakpeak aortic jet velocity
Progression of AA and AR dilation according to sex and valve phenotype can be seen in online supplemental figure 3.
Types of aortic valve fusion and aorta dilation
Among the 86 patients with BAV included in the present study, fusion type was available in 80 patients (two sinus BAV n=5; right-left coronary fusion n=56, right non-coronary fusion n=13; left non-coronary fusion n=6). There was no significant difference in AA dilation rate between fusion types (p=0.142; figure 2 panel B). AS severity progression rates were also similar between cusp fusion types (p=0.540). The same analysis was conducted for aortic root (AR) dimensions (online supplemental figure 2), and no significant differences were observed (p=0.960).
Clinical outcomes
During a median follow-up of 4.01 (95% CI 2.26 to 5.00) years, 121 (37%) patients underwent AVR (92 (76%) TAV and 29 (24%) BAV), 55 (17%) died (50 (91%) TAV and 5 (9%) BAV) and 159 (128 (80%) TAV and 31 (20%) BAV) patients met the composite endpoint of AVR or all-cause mortality. Of those who underwent AVR, 21 had combined AVR and AA interventions (9 TAV and 12 BAV). No aortic dissection occurred, and no patient underwent surgery for isolated AA intervention. Factors associated with the composite endpoint of death or AVR are presented in table 5. In univariate and multivariate analysis, only age was significantly associated with the composite clinical endpoint (p<0.001). After multivariate adjustments, aortic valve phenotype was not significantly associated with the risk of clinical events.
Table 5. Univariate and multivariate cox proportional hazard regression model for factors associated with the composite endpoint of aortic valve replacement and death.
| Univariate model | Multivariate model | |||
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age, per year increase | 1.02 (1.01 to 1.03) | <0.001 | 1.03 (1.01 to 1.05) | 0.003 |
| Female sex | 0.80 (0.55 to 1.16) | 0.226 | 0.88 (0.59 to 1.30) | 0.515 |
| Bicuspid aortic valve | 0.73 (0.49 to 1.08) | 0.099 | 1.43 [0.78 to 2.63) | 0.245 |
| AA diameter | 1.01 (0.98 to 1.05) | 0.413 | 1.00 (0.97 to 1.04) | 0.830 |
| Fast progression of AA dilation | 0.84 (0.61 to 1.15) | 0.271 | 0.91 (0.65 to 1.25) | 0.550 |
Fast progression of AA dilation=annualised progression above 0.18 mm/year. Values in bold reach statistical significance (p<0.05).
AAascending aorta HRhazard ratio
Discussion
The main findings of this study are that (1) BAV is associated with faster dilation of the AA but not of the AR; (2) factors associated with faster AA dilation are different between valve phenotype and sex: higher Apo B/Apo A-I ratio, higher baseline Vpeak and smaller baseline AA diameter are associated with faster AA dilation in the TAV cohort versus absence of hypertension, higher LDL levels and smaller baseline AA diameter in the BAV cohort; (3) predictors of AA dilation were different between sexes, with LDL levels for women and baseline Vpeak and AA dimensions for men.
Differences between baseline characteristics (age, sex, comorbidities, etc) of patients with BAV and TAV were expected since BAV with AS is a congenital heart disease that occurs earlier in life and is more prevalent in men versus women. Our study confirms the results of a previous study10 showing that BAV is associated with faster AA dilation compared with TAV. Furthermore, patients with BAV had larger AA diameters at baseline; hence, for these two reasons, patients with BAV may reach AA dilation thresholds proposed in the guidelines to trigger intervention earlier than in patients with TAV. In previous studies, BAV fusion type has been associated with the type and progression rate of aortopathy.23 24 In the present study, we did not find an association between BAV fusion type and AA dilation rate, but this analysis was limited by the small number of patients in some BAV fusion subtypes.
The counterintuitive inverted association between hypertension and AA dilation may be explained by the fact that patients with a diagnosis of hypertension are generally treated with medications targeting the renin-angiotensin system, which have been shown to be protective for aorta dilation in different diseases, including Marfan syndrome.25 26
The most intriguing finding of this study is the association between circulating lipoproteins and faster AA dilation, particularly in patients with BAV. Other previous studies also reported that higher levels of triglyceride-rich lipoproteins27 or cholesterol28 are associated with AA dilation in patients with BAV. It is also of note that patients with BAV in this study had significantly less hypolipemic treatment than patients with TAV, without being above the treatment threshold. Thus, patients with BAV had higher LDL and triglyceride levels than patients with TAV, who were more likely to have hypolipidemic treatment. A recent clinical trial has, however, shown that treatment with atorvastatin was inefective in reducing the progression of AA dilation.29
The previously mentioned association between lipoproteins and AA dilation was most notable in women, suggesting that lipoprotein management could be crucial in women, particularly.
Clinical implications
The results of this study suggest that treating comorbidities and in particular dyslipidemia, may help to prevent or slow AA dilation in patients with BAV. In our cohort, patients with BAV had mean LDL levels of 2.65±0.85 mmol/L and thus the vast majority are therefore within the target for primary prevention (<4.6 mmol/L) and would not require lipid-lowering therapy according to guidelines.30 However, in light of the results of this study and others, more aggressive targets, that is, targets for secondary prevention (<1.8 mmol/L), should be considered in patients with BAV.
Study limitations
The number of patients included in our analysis is higher than what was published in previous studies but still remains low. This could limit the statistical power of our analysis, specifically when analysing subgroups of patients. The absence of differences in AA dilation found between valve fusion types could be explained by this low number of patients. Furthermore, the results presented in this study only apply to patients with AS and cannot be extrapolated to all patients with BAV with normofunctional valves or with significant aortic regurgitation. Additional analysis should be done in this specific group of patients to evaluate the factors associated with AA dilation. In addition, we used echocardiographic parameters to measure AA diameter, but this imaging technique is not optimal for measuring the aorta. MRI or contrast computed tomography (CT) scans, the gold standard for measuring the aorta, were not available for all patients and therefore could not be used in this study.
Conclusion
This study suggests that BAV is associated with faster dilation of the AA. Predictors of AA dilation are different between valve phenotype and sex, with higher LDL levels and ApoB/Apo A-I ratios being associated with faster AA dilation in BAV and TAV, respectively. These findings provide support for the aggressive management of dyslipidemia in patients with BAV to prevent or slow the progression of AA dilation.
supplementary material
Acknowledgements
The authors of this study would like to thank Isabelle Fortin, Anne-Marie Doucet, Catherine Pichette, Anne-Élisabeth Roy-Pothier and Martine Parent for their help in data collection and management.
Footnotes
Funding: PP is funded by the Canada research Chair in Valvular Heart Diseases, Canadian Institutes of Helath research (#FDN-143225, Ottawa, Ontario, Canda).
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not applicable.
Ethics approval: This study involves human participants and was approved by Quebec Heart and Lung Institute Ethics Committee. Participants gave informed consent to participate in the study before taking part.
Data availability free text: Data are available on reasonable request. The datasets generated and analysed during the current study are not publicly available. Data are available from the corresponding author on reasonable request.
Contributor Information
Marie-Ange Fleury, Email: marie-ange.fleury.1@ulaval.ca.
Lionel Tastet, Email: lionel.tastet@criucpq.ulaval.ca.
Jérémy Bernard, Email: jeremy.bernard@criucpq.ulaval.ca.
Mylène Shen, Email: mylene.shen.1@ulaval.ca.
Romain Capoulade, Email: romain.capoulade@univ-nantes.fr.
Kathia Abdoun, Email: kathia.abdoun.1@ulaval.ca.
Élisabeth Bédard, Email: Elisabeth.Bedard@criucpq.ulaval.ca.
Marie Arsenault, Email: Marie.Arsenault@criucpq.ulaval.ca.
Philippe Chetaille, Email: philippe.chetaille.med@ssss.gouv.qc.ca.
Jonathan Beaudoin, Email: jonathan.beaudoin@criucpq.ulaval.ca.
Mathieu Bernier, Email: Mathieu.Bernier@criucpq.ulaval.ca.
Erwan Salaun, Email: erwan.salaun.1@ulaval.ca.
Nancy Côté, Email: nancy.cote@criucpq.ulaval.ca.
Philippe Pibarot, Email: philippe.pibarot@med.ulaval.ca.
Sébastien Hecht, Email: sebastien.hecht.1@ulaval.ca.
Data availability statement
Data are available upon reasonable request.
References
- 1.Ward C. Clinical significance of the bicuspid aortic valve. Heart. 2000;83:81–5. doi: 10.1136/heart.83.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roberts WC. The structure of the aortic valve in clinically isolated aortic stenosis: an autopsy study of 162 patients over 15 years of age. Circulation. 1970;42:91–7. doi: 10.1161/01.cir.42.1.91. [DOI] [PubMed] [Google Scholar]
- 3.Roberts C. The congenitally bicuspid aortic valve: a study of 85 autopsy cases. Am J Cardiol. 1970;26:72–83. doi: 10.1016/0002-9149(70)90761-7. [DOI] [PubMed] [Google Scholar]
- 4.Stock S, Mohamed SA, Sievers HH. Bicuspid aortic valve related aortopathy. Gen Thorac Cardiovasc Surg. 2019;67:93–101. doi: 10.1007/s11748-017-0821-x. [DOI] [PubMed] [Google Scholar]
- 5.Lindman BR, Clavel M-A, Mathieu P, et al. Calcific aortic stenosis. Nat Rev Dis Primers. 2016;2:16006. doi: 10.1038/nrdp.2016.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shang M, Kahler-Quesada A, Mori M, et al. Progression of aortic stenosis in patients with bicuspid aortic valve. J Card Surg. 2021;36:4665–72. doi: 10.1111/jocs.16026. [DOI] [PubMed] [Google Scholar]
- 7.Michelena HI, Khanna AD, Mahoney D, et al. Incidence of aortic complications in patients with bicuspid aortic valves. JAMA. 2011;306:1104–12. doi: 10.1001/jama.2011.1286. [DOI] [PubMed] [Google Scholar]
- 8.Wang J, Deng W, Lv Q, et al. Aortic Dilatation in Patients With Bicuspid Aortic Valve. Front Physiol. 2021;12:615175. doi: 10.3389/fphys.2021.615175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Girdauskas E, Borger MA, Secknus MA, et al. Is aortopathy in bicuspid aortic valve disease a congenital defect or a result of abnormal hemodynamics? A critical reappraisal of a one-sided argument. Eur J Cardiothorac Surg. 2011;39:809–14. doi: 10.1016/j.ejcts.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Kerneis C, Pasi N, Arangalage D, et al. Ascending aorta dilatation rates in patients with tricuspid and bicuspid aortic stenosis: the COFRASA/GENERAC study. Eur Heart J Cardiovasc Imaging. 2018;19:792–9. doi: 10.1093/ehjci/jex176. [DOI] [PubMed] [Google Scholar]
- 11.La Canna G, Ficarra E, Tsagalau E, et al. Progression rate of ascending aortic dilation in patients with normally functioning bicuspid and tricuspid aortic valves. Am J Cardiol. 2006;98:249–53. doi: 10.1016/j.amjcard.2006.01.096. [DOI] [PubMed] [Google Scholar]
- 12.Isselbacher EM, Preventza O, Hamilton Black J, 3rd, et al. 2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation. 2022;146:e334–482. doi: 10.1161/CIR.0000000000001106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borger MA, Fedak PWM, Stephens EH, et al. The American Association for Thoracic Surgery consensus guidelines on bicuspid aortic valve-related aortopathy: Full online-only version. J Thorac Cardiovasc Surg. 2018;156:e41–74. doi: 10.1016/j.jtcvs.2018.02.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shen M, Tastet L, Capoulade R, et al. Determinants of Aortic Stenosis Progression in Bicuspid and Tricuspid Aortic Valves. CJC Pediatr Congenit Heart Dis. 2022;1:184–92. doi: 10.1016/j.cjcpc.2022.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tastet L, Shen M, Capoulade R, et al. Sex Differences in the Progression of Aortic Valve Calcification and Clinical Outcomes: The PROGRESSA Study. JACC Cardiovasc Imaging. 2022;15:1349–51. doi: 10.1016/j.jcmg.2022.02.015. [DOI] [PubMed] [Google Scholar]
- 16.Tastet L, Shen M, Capoulade R, et al. Bone Mineral Density and Progression Rate of Calcific Aortic Valve Stenosis. J Am Coll Cardiol. 2020;75:1725–6. doi: 10.1016/j.jacc.2020.01.053. [DOI] [PubMed] [Google Scholar]
- 17.Michelena HI, Della Corte A, Evangelista A, et al. International consensus statement on nomenclature and classification of the congenital bicuspid aortic valve and its aortopathy, for clinical, surgical, interventional and research purposes. Eur J Cardiothorac Surg. 2021;60:448–76. doi: 10.1093/ejcts/ezab038. [DOI] [PubMed] [Google Scholar]
- 18.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 19.Goldstein SA, Evangelista A, Abbara S, et al. Multimodality imaging of diseases of the thoracic aorta in adults: from the American Society of Echocardiography and the European Association of Cardiovascular Imaging: endorsed by the Society of Cardiovascular Computed Tomography and Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr. 2015;28:119–82. doi: 10.1016/j.echo.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 20.Briand M, Dumesnil JG, Kadem L, et al. Reduced systemic arterial compliance impacts significantly on left ventricular afterload and function in aortic stenosis: implications for diagnosis and treatment. J Am Coll Cardiol. 2005;46:291–8. doi: 10.1016/j.jacc.2004.10.081. [DOI] [PubMed] [Google Scholar]
- 21.Garcia D, Pibarot P, Dumesnil JG, et al. Assessment of aortic valve stenosis severity: A new index based on the energy loss concept. Circulation. 2000;101:765–71. doi: 10.1161/01.cir.101.7.765. [DOI] [PubMed] [Google Scholar]
- 22.Hickey GL, Mokhles MM, Chambers DJ, et al. Statistical primer: performing repeated-measures analysis. Interact Cardiovasc Thorac Surg. 2018;26:539–44. doi: 10.1093/icvts/ivy009. [DOI] [PubMed] [Google Scholar]
- 23.Kusner JJ, Brown JY, Gleason TG, et al. The Natural History of Bicuspid Aortic Valve Disease. Struct Heart. 2023;7:100119. doi: 10.1016/j.shj.2022.100119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mahadevia R, Barker AJ, Schnell S, et al. Bicuspid aortic cusp fusion morphology alters aortic three-dimensional outflow patterns, wall shear stress, and expression of aortopathy. Circulation. 2014;129:673–82. doi: 10.1161/CIRCULATIONAHA.113.003026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brooke BS, Habashi JP, Judge DP, et al. Angiotensin II blockade and aortic-root dilation in Marfan’s syndrome. N Engl J Med. 2008;358:2787–95. doi: 10.1056/NEJMoa0706585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mullen M, Jin XY, Child A, et al. Irbesartan in Marfan syndrome (AIMS): a double-blind, placebo-controlled randomised trial. The Lancet. 2019;394:2263–70. doi: 10.1016/S0140-6736(19)32518-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Antequera-González B, Faiges M, Martínez-Micaelo N, et al. Glycoprotein and Lipoprotein Profiles Assessed by 1H-NMR and Its Relation to Ascending Aortic Dilatation in Bicuspid Aortic Valve Disease. J Clin Med. 2022;12:332. doi: 10.3390/jcm12010332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alegret JM, Masana L, Martinez-Micaelo N, et al. LDL cholesterol and apolipoprotein B are associated with ascending aorta dilatation in bicuspid aortic valve patients. QJM. 2015;108:795–801. doi: 10.1093/qjmed/hcv032. [DOI] [PubMed] [Google Scholar]
- 29.Evangelista A, Galian-Gay L, Guala A, et al. Atorvastatin Effect on Aortic Dilatation and Valvular Calcification Progression in Bicuspid Aortic Valve (BICATOR): A Randomized Clinical Trial. Circulation. 2024;149:1938–48. doi: 10.1161/CIRCULATIONAHA.123.067537. [DOI] [PubMed] [Google Scholar]
- 30.Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e596–646. doi: 10.1161/CIR.0000000000000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request.