Abstract
Background
Insertable cardiac monitor implantation is a simple and safe procedure commonly performed in patients with embolic stroke with undetermined source. Routine periprocedural antibiotic use is not recommended, because infection rate is very low, although some local infection or gram-positive bacteremia have been reported. We report a case of Pseudomonas monteilii sepsis immediately after insertable cardiac monitor implantation.
Case presentation
A 55-year-old Korean male with embolic stroke of undetermined source presented with gram-negative sepsis immediately after implantable cardiac monitor implantation as a first reported complication after the procedure. Pseudomonas monteilii was identified in the blood culture, and no other infection source was seen. He was treated with intravenous antibiotics without removing the device.
Conclusions
Prompt diagnosis and adequate management is required in such a patient with sepsis post-insertable cardiac monitor implantation procedure. It can be managed with adequate antibiotic treatment without device removal if there is no sign of inflammation at the insertion site. Further reports or studies should be investigated to reinforce this finding.
Learning objectives
The infection rate after insertable cardiac monitor insertion is extremely low; however, sepsis may occur without pocket infections. Physicians should be aware of signs of systemic infection, particularly when the procedure is performed outside the catheterization room. Sepsis after insertable cardiac monitor implantation can be managed with adequate antibiotic treatment without device removal if there is no sign of inflammation at the insertion site.
Keywords: Electrocardiography, Wearable electronic devices, Embolic stroke, Sepsis, Pseudomonas monteilii
Background
In patients with embolic stroke of undetermined source (ESUS), the current guideline includes a Class IIa recommendation for long-term cardiac rhythm monitoring [1]. Insertable cardiac monitor (ICM) effectively detects hidden atrial fibrillation after ESUS [2]. The ICM implantation procedure is minimally invasive, and trivial complications have been reported [3]. Traditionally, most ICM procedures have been performed in the cardiac catheterization lab; however, recently, many patients safely have undergone procedures outside the catheterization lab [4]. Due to its safety profile, it is easy for physicians to overlook checking for complications. However, as ICM insertion increases, infection related to ICM insertion has also been reported [3]. To our knowledge, sepsis caused by Gram-negative bacteria related to ICM insertion has been not reported yet. We report a case of a 55-year-old man with a serious complication due to Pseudomonas montelli after ICM implantation following ESUS.
Case presentation
A 55-year-old Korean man with no relevant medical history presented to hospital with dizziness and right hypoesthesia for 2 hours. He was a non-smoker and social drinker and had no family history of stroke. On neurological examination, a tilting tendency to the right side and left-beating torsional nystagmus were observed. His body temperature was 36.1 °C, his heart rate was 60 beats per minute, his respiration rate was 20 breaths per minute, and his blood pressure was 117/70 mmHg. He had a Glasgow Coma Scale score of 15 (E4V5M6; eye opening 4 points, verbal response 5 points, and motor response 6 points). Diffusion-weighted magnetic resonance (MR) imaging revealed acute infarction in the right lateral medulla. In addition, old ischemic lesions, presumed to be asymptomatic, were found in the left frontal lobe and right cerebellum (Fig. 1). MR angiography did not reveal any significant stenosis. He was admitted to the neurological department and started on antithrombotic therapy, including aspirin and clopidogrel. The mechanism of cerebral infarction was assumed to be ESUS because electrocardiography (ECG), Holter monitoring, transthoracic echocardiography, and transesophageal echocardiography performed within 3 days of symptom onset demonstrated nonspecific findings. We decided to insert the ICM to detect hidden atrial fibrillation according to current guidelines.
Fig. 1.

Neuroimaging of the brain. A Diffusion-weighted magnetic resonance (MR) imaging demonstrated a small ischemic lesion in the right lower lateral medulla. Old ischemic lesion in the right cerebellum (C, fluid-attenuated inversion recovery image) as well as in the left cingulate gyrus (B, T1 weighted image). The arrows represent the regions affected by the embolic infarct. (D and E) There were no abnormal findings on MR angiography; thus, an embolic stroke of undetermined source was the presumed etiology of ischemic stroke.
The procedure was performed at the 10th hospital day. After administering chlorohexidine in the cardiac catheterization room, the Reveal XT/LINQ™ (Medtronic, Minneapolis, MN, USA) ICM device was inserted within a few minutes. After a 2-point suture, the procedure was completed with gauze dressing. No immediate procedure-related complications occurred. His hemoglobin A1c level was 6.4%, and serology for human immunodeficiency virus before the procedure showed negative.
A total of 21 hours after the procedure, the patient developed a high fever of up to 40.2 °C and chills. On examination, blood pressure was 116/82 mmHg, his heart rate was 99 beats per minute, and oxygen saturation was 95%. Quick sepsis-related organ failure assessment (SOFA) score for sepsis was 1 point, with his respiration rate 24 beats per minute, and Glasgow Coma Scale score was 15 points. Laboratory results were notable, with a white cell count of 8200/μl (reference range, 4500–11,000), C-reactive protein level of 14 mg/dl (reference range, less than 0.3), and procalcitonin of 28 ng/ml (reference range, less than 0.1). The incision site of the ICM showed no signs of inflammation, such as swelling, erythema, tenderness, or discharge (Fig. 2). Aerobic and anaerobic bacteria did not grow in cultures performed at the incision site. Screening tests for coronavirus disease 2019 and influenza viruses yielded negative results. There was no erythema or tenderness at the intravenous injection site that was inserted 4 days before the procedure. Routine urinalysis did not reveal pyuria or bacteriuria. Chest and abdominal computed tomography revealed, there was unremarkable findings except for mild bladder wall thickening (Fig. 3). Cefazolin as an empirical antibiotic was administered under suspicion of procedure-related bacteremia. A gram-negative rod was identified in the interim report of the blood culture 2 days later. Pseudomonas monteilii was identified in two sets of blood cultures, and a susceptible antibiotic, cefepime, was administered for 2 weeks. The patient was discharged without complications and followed up in the outpatient department without fever for 2 years. The ICM was removed after 1 year of outpatient follow-up because the patient wanted it removed before the battery ran out. In the 1-year analysis, no significant arrhythmias including atrial fibrillation were detected. He was prescribed aspirin and clopidogrel for 6 months, then switched to clopidogrel, and there was no recurrence of stroke during the follow-up.
Fig. 2.

A Insertable cardiac monitor insertion site of the patient. There was no sign of inflammation, such as erythema, swelling, or purulent discharge at the incision site. B Chest x-ray of the patient after the procedure. The location of the ICM was on the left chest wall
Fig. 3.
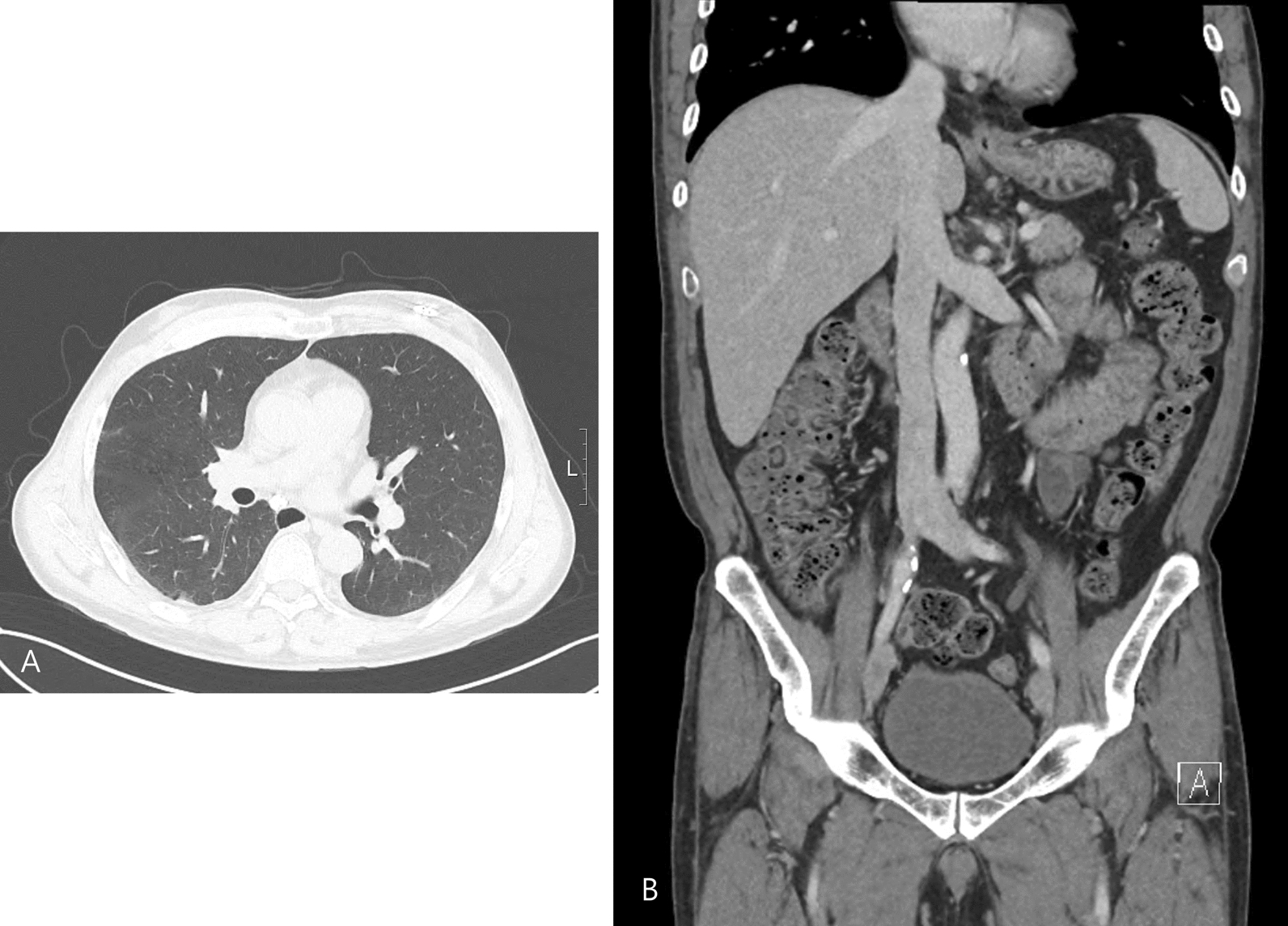
(A) Chest and (B) abdomen computed tomography of the patient after fever developed. There was a nonspecific finding without mild bladder wall thickening
Discussion and conclusions
To our knowledge, this is the first report of gram-negative bacterial sepsis due to Pseudomonas monteilii in a patient with ESUS after ICM insertion. Only one case of bacteremia has been reported after ICM insertion, and the causative organism was Staphylococcus aureus [3]. Bacteremia is more common in cardiac implantable electronic devices, including permanent pacemakers and transvenous implantable cardioverter defibrillators, because the lead is inserted directly into the venous system. Most cases of gram-negative bacteremia in cardiac implantable electronic device patients were not related to device [5]. In this case, the patient suffered from Pseudomonas monteilii sepsis just after ICM insertion and was discharged without the removal of the device.
The method of confirming the route of infection is that the same organism grows at the implanted device, and isolated organisms have the same genetic information. Because he was treated without removing the device, a definitive diagnosis of infection source could not be made. However, he had no neurologic sequalae after the stroke, and no other procedure including intravenous line insertion was carried out before or after implantation. Considering these clinical situations, it would be reasonable to believe the bacteremia is related to the insertion of ICM. A molecular test of the bacteria was not carried out, because his fever and general condition improved after susceptible antibiotics administration.
As the indications for ICM are widening, it is used not only for ESUS but also for large- or small-vessel disease and unexplained syncope [6, 7]. Complications of ICM insertion include insertion site pain, major or minor bleeding, and wound infection or pocket erosion [8]. Because the infection rate was very low, a registry study did not show an increase in infections without prophylactic antibiotics [9]. The author investigated 137 previously ICM-implanted patients, and no local or systemic infections requiring device removal were found [10].
The risk factors for infection with cardiac-implantable electronic devices are diabetes mellitus and underlying heart disease [11]. Early onset cardiac implantable electronic device infections frequently originate from generator pocket contamination. Hematogenous seeding from a remote source of bacteremia often leads to device infection [12]. Deciding to remove or retain the device without evidence of pocket infection is difficult. Similar to ICMs, subcutaneous-implantable cardioverter defibrillators are located in the subcutaneous layer. Many infection cases were managed without device removal, and Staphylococcus species were the most common cause of bacteremia [13]. In this case, the fever resolved 3 days after antibiotic administration, and the patient was discharged without device removal.
Pseudomonas monteilii, which belongs to the Pseudomonas putida phylogenetic group, was first isolated in 1997 [14]. It is an uncommon species of Pseudomonas, and it is often found in healthcare environments [15]. Previous case reports have described an organism causing pneumonia or meningitis [16, 17]; however, no procedure-related infections have been reported. In this case, there were post-procedural fever and bacteremia, but we could not identify the source of infection on abdominal and chest CT scans. Although there is a possibility of opportunistic infection or intravenous injection site infection, it is very unlikely because he was immunocompetent, and intravenous line had been changed a day before the procedure. Insertion procedure was performed with routine aseptic technique in the catheterization lab, although there may have been contamination that we were not aware of. We concluded that it was a procedure-related infection and treated it with susceptible antibiotics. Considering the potential for Pseudomonas monteilii to colonize hospital environments, it is possible that the patient’s skin flora caused the infection during the procedure, or it could have been transmitted through the healthcare staff’s hands. This highlights the importance of aseptic techniques during procedures and appropriate hospital infection control measures.
When fever occurs after ICM insertion, even if the risk of infection is very low, gram-negative bacterial sepsis should be considered as a possible cause. We suggest that immediate bacteremia following a procedure can be treated with antibiotics without the need for device removal. However, this finding should be further investigated through well-structured studies.
Acknowledgements
None.
Abbreviations
- CT
Computed tomography
- ECG
Electrocardiography
- ESUS
Embolic stroke of undetermined source
- ICM
Insertable cardiac monitor
- MR
Magnetic resonance
Author contributions
Conception, design, draft, and revision: Dongwhane Lee and In Tae Moon. Acquisition, analysis, and interpretation of data: Kyung Hwa Jung and Hyo-Ju Son.
Funding
None.
Availability of data and materials
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
Declarations
Ethics approval and consent to participate
This study has been approved by the Uijeongbu Eulji Medical Center (Institutional Review Board approval number: 2023–09-011).
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing of interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kleindorfer DO, Towfighi A, Chaturvedi S, Cockroft KM, Gutierrez J, Lombardi-Hill D, et al. 2021 guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American heart association/American stroke association. Stroke. 2021;52(7):e364–467. [DOI] [PubMed] [Google Scholar]
- 2.Sanna T, Diener HC, Passman RS, Di Lazzaro V, Bernstein RA, Morillo CA, et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. 2014;370(26):2478–86. [DOI] [PubMed] [Google Scholar]
- 3.Diederichsen SZ, Haugan KJ, Højberg S, Holst AG, Køber L, Pedersen KB, et al. Complications after implantation of a new-generation insertable cardiac monitor: Results from the LOOP study. Int J Cardiol. 2017;241:229–34. [DOI] [PubMed] [Google Scholar]
- 4.Beinart SC, Natale A, Verma A, Amin A, Kasner S, Diener HC, et al. Real-world comparison of in-hospital Reveal LINQ insertable cardiac monitor insertion inside and outside of the cardiac catheterization or electrophysiology laboratory. Am Heart J. 2019;207:76–82. [DOI] [PubMed] [Google Scholar]
- 5.Uslan DZ, Sohail MR, Friedman PA, Hayes DL, Wilson WR, Steckelberg JM, et al. Frequency of permanent pacemaker or implantable cardioverter-defibrillator infection in patients with gram-negative bacteremia. Clin Infect Dis. 2006;43(6):731–6. [DOI] [PubMed] [Google Scholar]
- 6.Bernstein RA, Kamel H, Granger CB, Piccini JP, Sethi PP, Katz JM, et al. Effect of long-term continuous cardiac monitoring vs usual care on detection of atrial fibrillation in patients with stroke attributed to large- or small-vessel disease: The STROKE-AF randomized clinical trial. JAMA. 2021;325(21):2169–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brignole M, Moya A, de Lange FJ, Deharo JC, Elliott PM, Fanciulli A, et al. 2018 ESC Guidelines for the diagnosis and management of syncope. Eur Heart J. 2018;39(21):1883–948. [DOI] [PubMed] [Google Scholar]
- 8.Mittal S, Sanders P, Pokushalov E, Dekker L, Kereiakes D, Schloss EJ, et al. Safety profile of a miniaturized insertable cardiac monitor: results from two prospective trials. Pacing Clin Electrophysiol. 2015;38(12):1464–9. [DOI] [PubMed] [Google Scholar]
- 9.Beinart SC, Natale A, Verma A, Amin A, Kasner S, Diener HC, et al. Real-world use of prophylactic antibiotics in insertable cardiac monitor procedures. Pacing Clin Electrophysiol. 2016;39(8):837–42. [DOI] [PubMed] [Google Scholar]
- 10.Lee JH, Moon IT, Cho Y, Kim JY, Kang J, Kim BJ, et al. Left atrial diameter and atrial ectopic burden in patients with embolic stroke of undetermined source: risk stratification of atrial fibrillation with insertable cardiac monitor analysis. J Clin Neurol (Seoul, Korea). 2021;17(2):213–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hercé B, Nazeyrollas P, Lesaffre F, Sandras R, Chabert JP, Martin A, et al. Risk factors for infection of implantable cardiac devices: data from a registry of 2496 patients. Europace. 2013;15(1):66–70. [DOI] [PubMed] [Google Scholar]
- 12.DeSimone DC, Sohail MR. Management of bacteremia in patients living with cardiovascular implantable electronic devices. Heart Rhythm. 2016;13(11):2247–52. [DOI] [PubMed] [Google Scholar]
- 13.Gold MR, Aasbo JD, Weiss R, Burke MC, Gleva MJ, Knight BP, et al. Infection in patients with subcutaneous implantable cardioverter-defibrillator: Results of the S-ICD Post Approval Study. Heart Rhythm. 2022;19(12):1993–2001. [DOI] [PubMed] [Google Scholar]
- 14.Elomari M, Coroler L, Verhille S, Izard D, Leclerc H. Pseudomonas monteilii sp nov, isolated from clinical specimens. Int J Syst Bacteriol. 1997;47(3):846–52. [DOI] [PubMed] [Google Scholar]
- 15.de Abreu PM, Farias PG, Paiva GS, Almeida AM, Morais PV. Persistence of microbial communities including Pseudomonas aeruginosa in a hospital environment: a potential health hazard. BMC Microbiol. 2014;14:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aditi Shariff M, Beri K. Exacerbation of bronchiectasis by Pseudomonas monteilii: a case report. BMC Infect Dis. 2017;17(1):511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toledo H, Martín-Gutiérrez G, Lepe JA. Pseudomonas monteilii nosocomial meningitis in a patient with an intraventricular catheter. Enfermedades infecciosas y microbiologia clinica (English ed). 2021. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.


