Abstract
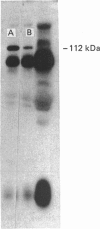
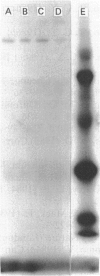
A hexose-transport regulatory mutant (D1/S4) was isolated from L6 rat myoblasts on the basis of its resistance to detachment and cell lysis in the presence of antibody and complement. Growth studies indicated that D1/S4 cells had a slower doubling time (29 h) compared with the parental L6 cells (22 h). Furthermore, after 9 days growth, less than 1% cell fusion was observed with D1/S4 cells, whereas 95% cell fusion was observed with the L6 cells. When the parental L6 cells were starved of glucose or treated with anti-L6 antibody, a significant increase in the Vmax, of 2-deoxy-D-glucose (dGlc) and 3-O-methyl-D-glucose (MeGlc) transport was observed. Although glucose-grown D1/S4 cells possessed normal hexose-transport activity, the above treatments had no effect on dGlc and MeGlc transport in these cells. Electrophoresis and immunoblotting studies revealed that D1/S4 cells possessed decreased amounts of a 112 kDa plasma-membrane protein. It is conceivable that this protein may play a role in triggering the antibody- and glucose-starvation-mediated activation of hexose transport and in myogenic differentiation. Unlike D1/S4, mutant F72, a mutant defective in the high-affinity hexose-transport system, was found to possess normal amounts of the 112 kDa protein. Although glucose starvation has no effect on the hexose-transport activity in this mutant, its hexose transport activity can be increased by antibody treatment. These studies with mutants suggest the involvement of regulatory components in the activation of hexose transport.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cheung M. O., Lo T. C. Hexose transport in plasma membrane vesicles of rat myoblast L6. Can J Biochem Cell Biol. 1984 Nov;62(11):1217–1227. doi: 10.1139/o84-156. [DOI] [PubMed] [Google Scholar]
- Christopher C. W., Kohlbacher M. S., Amos H. Transport of sugars in chick-embryo fibroblasts. Evidence for a low-affinity system and a high-affinity system for glucose transport. Biochem J. 1976 Aug 15;158(2):439–450. doi: 10.1042/bj1580439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopher C. W., Ullrey D., Colby W., Kalckar M. Paradoxical effects of cycloheximide and cytochalasin B on hamster cell hexose uptake. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2429–2433. doi: 10.1073/pnas.73.7.2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Amore T., Cheung M. O., Duronio V., Lo T. C. Stimulation of hexose transport in L6 rat myoblasts by antibody and by glucose starvation. Biochem J. 1986 Sep 15;238(3):831–836. doi: 10.1042/bj2380831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Amore T., Duronio V., Cheung M. O., Lo T. C. Isolation and characterization of hexose transport mutants in L6 rat myoblasts. J Cell Physiol. 1986 Jan;126(1):29–36. doi: 10.1002/jcp.1041260105. [DOI] [PubMed] [Google Scholar]
- D'Amore T., Lo T. C. Approaches used to examine the mechanism and regulation of hexose transport in rat myoblasts. Biochem Cell Biol. 1986 Nov;64(11):1081–1091. doi: 10.1139/o86-143. [DOI] [PubMed] [Google Scholar]
- D'Amore T., Lo T. C. Hexose transport in L6 rat myoblasts. I. Rate-limiting step, kinetic properties, and evidence for two systems. J Cell Physiol. 1986 Apr;127(1):95–105. doi: 10.1002/jcp.1041270113. [DOI] [PubMed] [Google Scholar]
- D'Amore T., Lo T. C. Hexose transport in L6 rat myoblasts. II. The effects of sulfhydryl reagents. J Cell Physiol. 1986 Apr;127(1):106–113. doi: 10.1002/jcp.1041270114. [DOI] [PubMed] [Google Scholar]
- Gay R. J., Hilf R. Density-dependent and adaptive regulation of glucose transport in primary cell cultures of the R3230AC rat mammary adenocarcinoma. J Cell Physiol. 1980 Feb;102(2):155–174. doi: 10.1002/jcp.1041020207. [DOI] [PubMed] [Google Scholar]
- Haspel H. C., Wilk E. W., Birnbaum M. J., Cushman S. W., Rosen O. M. Glucose deprivation and hexose transporter polypeptides of murine fibroblasts. J Biol Chem. 1986 May 25;261(15):6778–6789. [PubMed] [Google Scholar]
- Kalckar H. M., Ullrey D. B. Further clues concerning the vectors essential to regulation of hexose transport, as studied in fibroblast cultures from a metabolic mutant. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1126–1129. doi: 10.1073/pnas.81.4.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klip A., Rothstein A., Mack E. Stimulation of hexose uptake in rat thymic lymphocytes by phorbol ester. A role for Ca2+ and Na+/H+ exchange? Biochem Biophys Res Commun. 1984 Oct 15;124(1):14–22. doi: 10.1016/0006-291x(84)90909-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lo T. C., Duronio V. Activation of hexose transport by antibody. Can J Biochem Cell Biol. 1984 May;62(5):245–254. doi: 10.1139/o84-034. [DOI] [PubMed] [Google Scholar]
- Lo T. C., Duronio V. Mechanism of antibody stimulation of hexose transport in rat myoblasts. Can J Biochem Cell Biol. 1984 May;62(5):255–265. doi: 10.1139/o84-035. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Fox C. F. Surface-specific iodination of membrane proteins of viruses and eucaryotic cells using 1,3,4,6-tetrachloro-3alpha,6alpha-diphenylglycoluril. Biochemistry. 1978 Oct 31;17(22):4807–4817. doi: 10.1021/bi00615a031. [DOI] [PubMed] [Google Scholar]
- Morris G. E., Cole R. J. Cell fusion and differentiation in cultured chick muscle cells. Exp Cell Res. 1972 Nov;75(1):191–199. doi: 10.1016/0014-4827(72)90536-8. [DOI] [PubMed] [Google Scholar]
- Rapaport E., Christopher C. W., Svihovec S. K., Ullrey D., Kalckar H. M. Selective high metabolic lability of uridine, guanosine and cytosine triphosphates in response to glucose deprivation and refeeding of untransformed and polyoma virus-transformed hamster fibroblasts. J Cell Physiol. 1979 Nov;101(2):229–235. doi: 10.1002/jcp.1041010205. [DOI] [PubMed] [Google Scholar]
- Salter D. W., Baldwin S. A., Lienhard G. E., Weber M. J. Proteins antigenically related to the human erythrocyte glucose transporter in normal and Rous sarcoma virus-transformed chicken embryo fibroblasts. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1540–1544. doi: 10.1073/pnas.79.5.1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrey D., Gammon M. T., Kalckar H. M. Uptake patterns and transport enhancements in cultures of hamster cells deprived of carbohydrates. Arch Biochem Biophys. 1975 Apr;167(2):410–416. doi: 10.1016/0003-9861(75)90481-6. [DOI] [PubMed] [Google Scholar]
- Yaffe D. Retention of differentiation potentialities during prolonged cultivation of myogenic cells. Proc Natl Acad Sci U S A. 1968 Oct;61(2):477–483. doi: 10.1073/pnas.61.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Putten J. P., Krans H. M. Glucose as a regulator of insulin-sensitive hexose uptake in 3T3 adipocytes. J Biol Chem. 1985 Jul 5;260(13):7996–8001. [PubMed] [Google Scholar]