Fig. 2 │. GIST signalling pathways, drug targets and current systemic therapies.
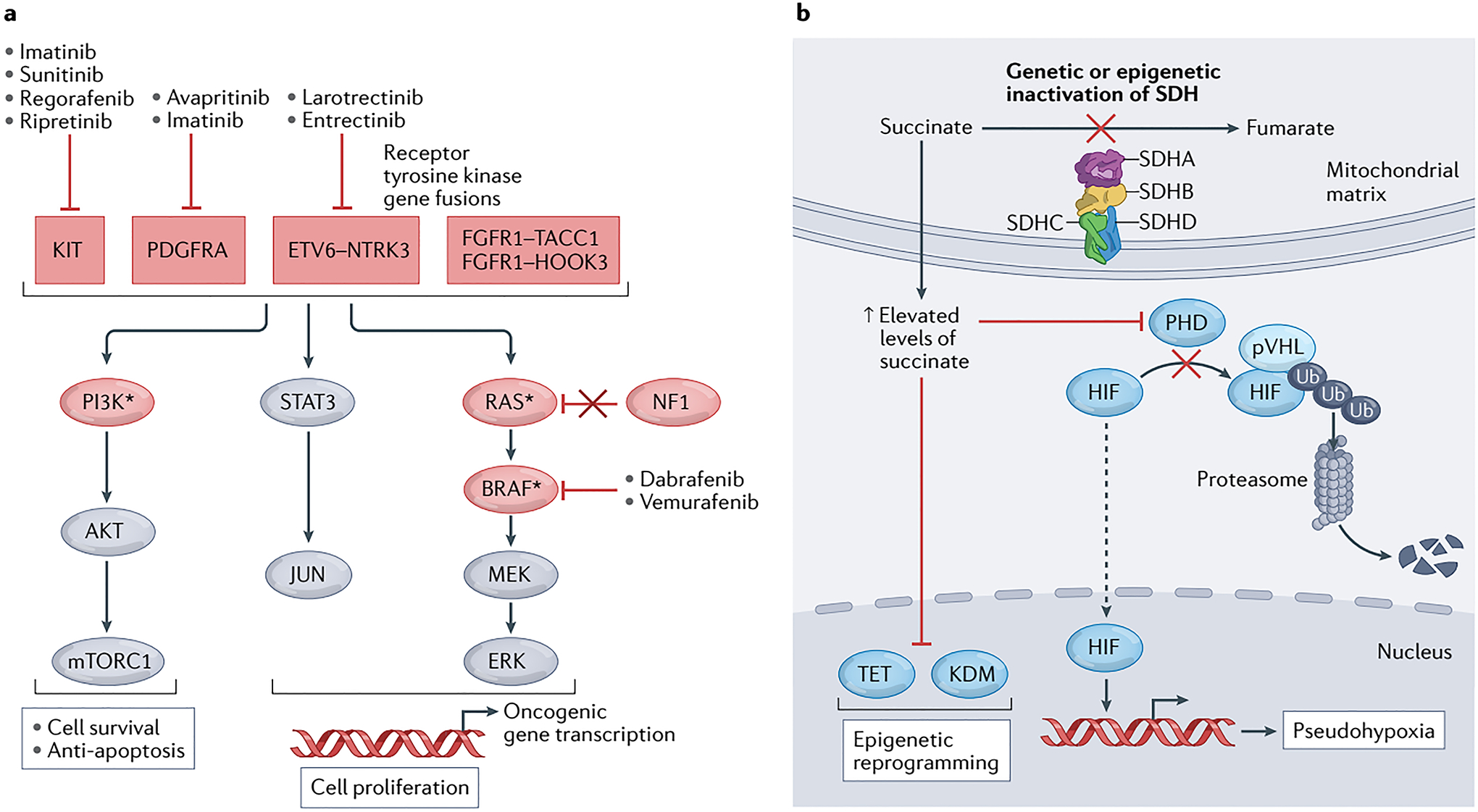
a │ The genetic alterations that drive gastrointestinal stromal tumours (GISTs) generally result in activation of signalling through the MEK–ERK (MAPK), JAK–STAT and PI3K–AKT pathways to prevent apoptosis and drive cell survival and proliferation. The components of these pathways and upstream receptor tyrosine kinases that are recurrently mutated in GIST are shown in red ovals and boxes, respectively; those indicated with an asterisk can also arise as secondary mutations after therapy. Gain-of-function (activating) mutations are found in positive signalling effectors, including KIT, PDGFRA, fusion proteins involving NTRK3 (TRKC) or FGFR1, as well as RAS, PI3Kα or BRAF. Loss-of-function (inactivating) mutations are found in tumour suppressors, such as neurofibromatosis-related protein NF1 (also known as neurofibromin). b │ GIST can also be driven by deficiency of the mitochondrial respiratory complex II, succinate dehydrogenase (SDH), resulting from a genetic mutation in any one of the four SDH subunit genes (SDHA, SDHB, SDHC or SDHD), or more rarely from epigenetic inactivation of SHDC via promoter hypermethylation. Inactivation of the SDH complex results in an accumulation of succinate, which leads to competitive inhibition of α-ketoglutarate-dependent dioxygenases, including those of the hypoxia-inducible factor (HIF)-prolyl hydroxylase domain (PHD), ten-eleven translocation methylcytosine dioxygenase (TET), and lysine-specific histone demethylase (KDM) families. In turn, inhibition of PHDs leads to pseudohypoxia by preventing von Hippel-Lindau disease tumour suppressor (pVHL)-mediated ubiquitination and subsequent proteasomal degradation of HIF, while inhibition of TET and KDM proteins results in increased methylation of DNA and histones, respectively, and thus broad epigenetic reprogramming. Ub, ubiquitin.
