Fig. 4 │. New therapeutic approaches exploiting different elements of GIST biology.
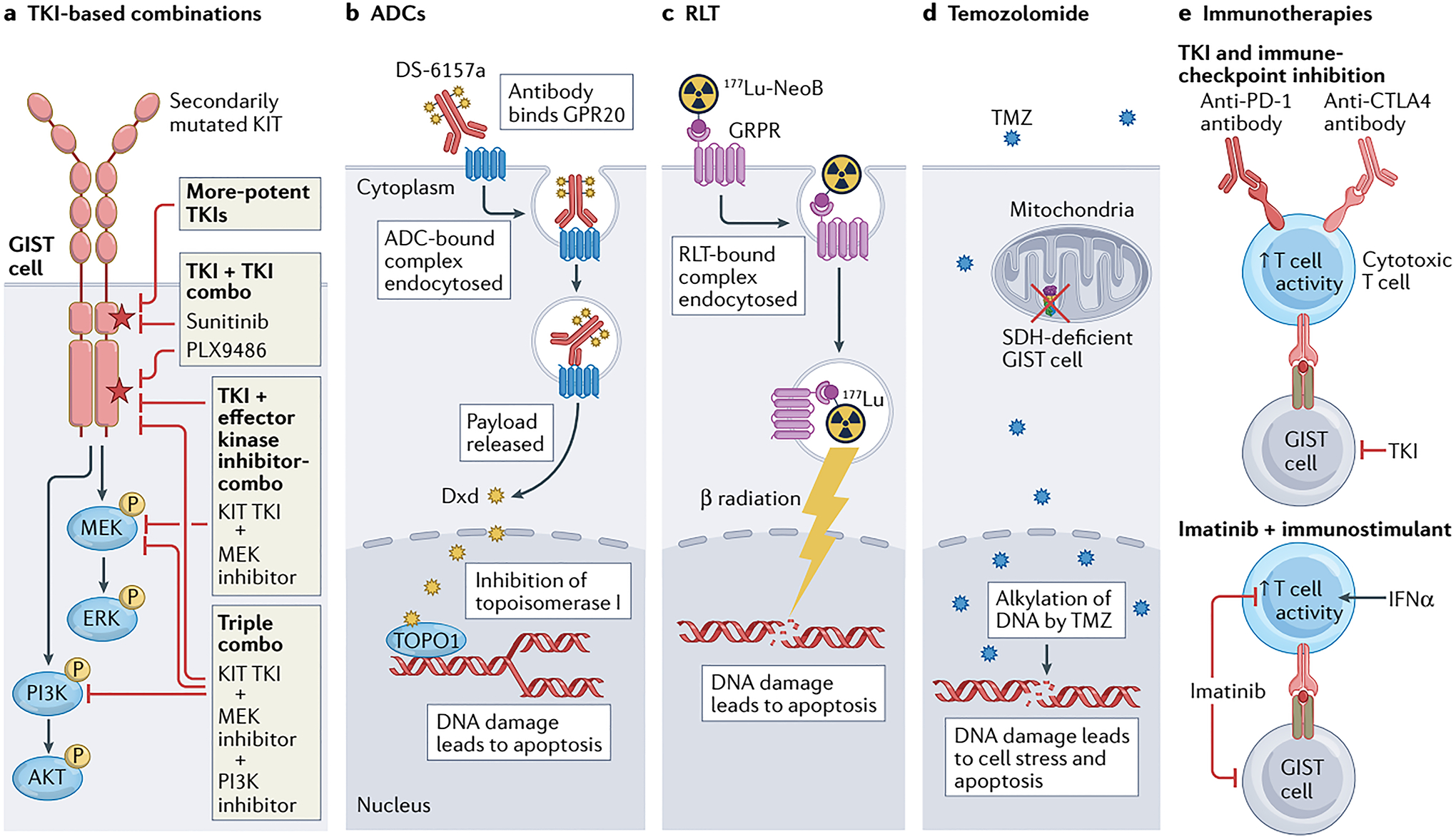
a │ Tyrosine kinase inhibitor (TKI)-based therapy remains relevant for the majority of patients with advanced-stage GIST, particularly those with KIT-mutant disease; however, new strategies are required to overcome treatment resistance and thereby improve outcomes, including the development of more-potent TKIs, or combinations of two TKIs or a TKI plus inhibitors of downstream effector kinases (such as MEK and/or PI3K). Many of these approaches might also be applicable in PDGFRA-mutant GIST if PDGFRA-specific TKIs, such as avapritinib, are utilized. b │ The antibody–drug conjugate (ADC) DS-6157a combines an anti-G protein-coupled receptor 20 (GPR20) antibody and the DNA topoisomerase I (TOPO1) inhibitor deruxtecan (Dxd). Upon binding to GPR20, the receptor–ADC complex is endocytosed, with subsequent lysosomal degradation of the complex resulting in release of the Dxd payload that in turn causes DNA damage and cell death. c │ 177Lu-NeoB is a radioligand therapy (RLT) consisting of the radioisotope 177Lu conjugated via the chelating agent dodecanetetraacetic acid (DOTA) to a peptide antagonist of the gastrin-releasing peptide receptor (GRPR; also known as bombesin receptor subtype 2 (BB2)). Thus, this agent enables specific intracellular delivery of radiation to GRPR-expressing GIST cells, resulting in DNA damage and apoptosis. d │ In an approach specific to SDH-deficient GIST cells, treatment with the alkylating agent temozolomide (TMZ) can cause irreparable DNA damage and cell death. This vulnerability is probably at least partially attributable to epigenetic silencing of 6-O-methylguanine-DNA methyltransferase (MGMT), which is involved in the repair of alkylated DNA, as a consequence of the metabolic alterations resulting from SDH deficiency in these cells. e │ Various immuno-oncology approaches to the treatment of GIST have been proposed, including combining a TKI with PD-1 and/or CTLA4 immune-checkpoint inhibitors to simultaneously suppress GIST cells while stimulating antitumour T cells, or imatinib with an immunostimulant such as IFNα to prevent imatinib-related T cell inactivation.
