Abstract
BACKGROUND
Injury to the internal carotid artery (ICA) during functional endoscopic sinus surgery is a rare but potentially fatal complication. Although treatment algorithms have been developed, guidelines for effectively managing iatrogenic ICA injury have not been established. A case of ICA perforation during functional endoscopic sinus surgery treated with cerebral bypass utilizing a cephalic vein graft is presented.
OBSERVATIONS
A woman in her late 50s presented with a left cavernous ICA injury that had occurred during endoscopic nasal polypectomy at an outside hospital. Hemostasis was achieved with intranasal Foley catheter placement. Left common carotid artery angiography revealed a high-flow carotid-cavernous fistula. Cerebral revascularization was chosen as the optimal procedure. The initial intent was to use a radial artery graft, but the radial artery was found to be occluded intraoperatively. Postoperatively, the patient experienced decreased vision and left eye movement but was otherwise neurologically intact. Postoperative angiography showed complete resolution of the fistula.
LESSONS
In cases involving ICA injury and carotid-cavernous fistula formation, microsurgical trapping with high-flow bypass is a favorable treatment option. The cephalic vein is a viable graft option when unexpected challenges arise with a radial artery graft.
Keywords: cephalic vein graft, endoscopic sinus surgery, extracranial-intracranial bypass, internal carotid artery injury
ABBREVIATIONS: ACA = anterior cerebral artery, CCA = common carotid artery, CCF = carotid-cavernous fistula, EESBS = endoscopic endonasal skull base surgery, FESS = functional endoscopic sinus surgery, ICA = internal carotid artery, MCA = middle cerebral artery.
Injury to the internal carotid artery (ICA) during endoscopic sinus surgery, including functional endoscopic sinus surgery (FESS) or endoscopic endonasal skull base surgery (EESBS), is a rare but potentially fatal complication, with an incidence of approximately 0.025% for FESS and between 0.2% and 1% for EESBS.1–6 ICA injury can lead to massive hemorrhage, stroke, shock, and death within minutes. Urgent management is essential to prevent life-threatening complications. The narrow and deep operative field in the nasal cavity makes management during endonasal surgery especially difficult.7 Even in the case of successful hemostasis, iatrogenic ICA injury can lead to a direct high-flow carotid-cavernous fistula (CCF).1 CCFs are an abnormal communication between the high-pressure arterial flow of the ICA and the low-pressure venous flow within the cavernous sinus, classically presenting with a triad of chemosis, exophthalmos, and ocular bruit.8–10 Although treatment algorithms have been developed, there are no established guidelines for effectively managing iatrogenic ICA injury.2, 4, 8, 11–13 Here, we describe a woman in her late 50s who presented with a high-flow CCF after iatrogenic ICA injury during FESS at an outside hospital.
Illustrative Case
History and Examination
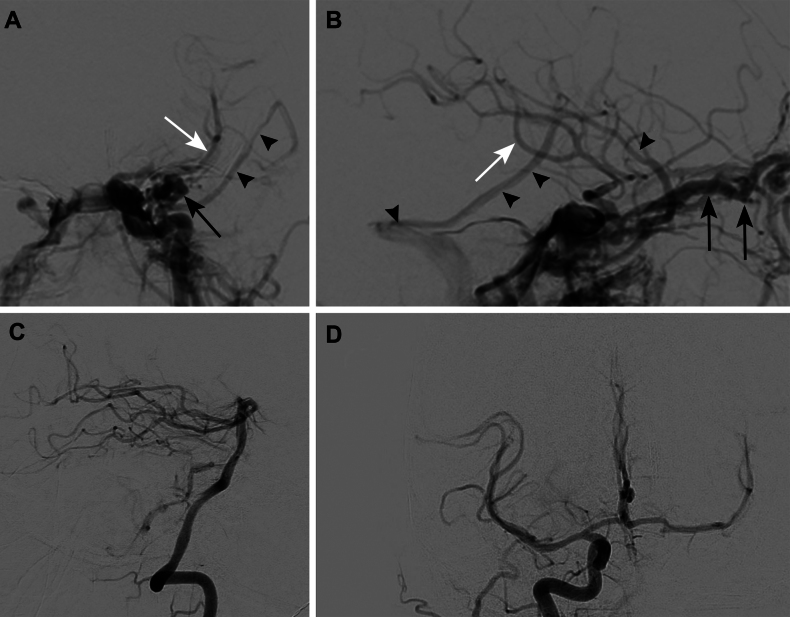
A woman in her late 50s presented with a left cavernous ICA injury that had occurred during an endoscopic nasal polypectomy at an outside hospital. Hemostasis was achieved with intranasal Foley catheter placement. Upon arrival at our facility, the patient was medically sedated and chemically paralyzed with evidence of chemosis and proptosis of the left eye. Normotension was maintained. The patient’s medical history included asthma, allergic rhinitis, recurrent sinusitis, obesity, hepatic steatosis, resolved Bell’s palsy, diverticulosis, and osteoarthritis. Left common carotid artery (CCA) angiography revealed a high-flow CCF. Cortical venous reflux into the superficial sylvian vein was also evident; this reflux drained into the vein of Labbé and transverse sinus (Fig. 1A and B). Angiography also showed dilation of the left ophthalmic vein and continued filling of the left middle cerebral artery (MCA) candelabra past the Foley balloon occlusion of the ICA. The left vertebral artery (Fig. 1C) and right CCA (Fig. 1D) injections showed no supplemental flow from the posterior circulation but robust flow across the anterior communicating artery. Magnetic resonance imaging (MRI) showed multiple scattered embolic infarcts (Fig. 2).
FIG. 1.
Preoperative diagnostic cerebral angiography. Anteroposterior (A) and lateral (B) views of the left CCA showing a high-flow CCF. Black arrows indicate the dilated left ophthalmic vein. Black arrowheads indicate cortical venous reflux into the superficial sylvian vein. White arrows indicate continued filling of the left MCA candelabra past the Foley balloon occlusion of the ICA. Left vertebral artery (C) and right CCA (D) injections demonstrate no supplemental flow from the posterior circulation and robust flow across the anterior communicating artery.
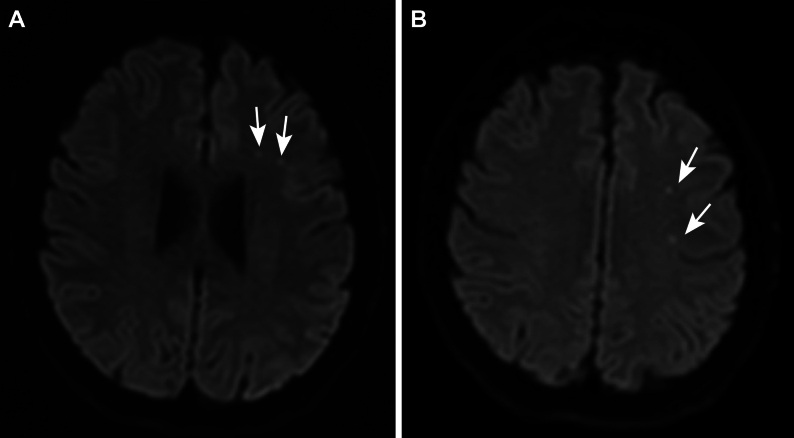
FIG. 2.
Axial MRI at the level of the lateral ventricles (A) and above the lateral ventricles (B) showing multiple scattered embolic infarcts in the left frontal lobe (white arrows).
After multidisciplinary discussion, it was decided that endovascular treatment with a covered stent or flow diverter and possible coiling would involve high risk due to continued bleeding with balloon takedown. Therefore, it was decided that surgical trapping of the ICA with extracranial-intracranial bypass was the optimal procedure. This procedure would allow the safe removal of the Foley balloon catheter and minimize the risk of intraoperative ischemia because of the robust blood flow observed across the anterior communicating artery. It would also allow complete ICA trapping to effectively address the fistula, preventing further recruitment of small direct feeders. Moreover, it would enable the surgical packing of the sinus and enhanced cerebral blood flow, offering protection against future hypoperfusion-related ischemic events, which is a protection that is not typically afforded by low-flow bypass procedures. The risks of this procedure included potential injury to cranial nerves II, III, X, and XII or the phrenic nerve, bleeding associated with the clinoidectomy, and graft occlusion after surgery.
Surgical Procedure
Equipment for the Procedure
The procedure required the use of microclips to ligate branches, a vessel pressure distension syringe tip, temporary and permanent aneurysm clips, 7-0 Prolene suture, 9-0 BV130-4 needles, a Leyla bar for retraction with fishhooks, a carotid endarterectomy set, a bypass set, a 4-mm aortic punch, an operating microscope with indocyanine green, and an endoscopic tower and endoscopic set.
Description of the Procedure
Three teams began the procedure: one performed ipsilateral carotid bifurcation exposure, another performed contralateral harvesting of the cephalic vein graft, and a third focused on the craniotomy. Once the cephalic vein was harvested, a Garrett line was drawn to visualize twisting and ensure proper graft positioning. Valve orientation was checked, with valves pointing from distal to proximal. Pressure distension under the microscope was performed, and side branches were ligated to prevent graft leakage.
A standard pterional craniotomy was performed, providing access to the anterior circulation. An extradural anterior clinoidectomy was necessary to allow proximal access to the intracranial ICA. A fibrin sealant was applied in the cavernous sinus to control brisk bleeding, and temporary clipping of the ICA in the neck was needed to temporarily control CCF bleeding.
Dural opening, with wide dissection of the sylvian fissure, and distal dural ring takedown were performed. The M2 (insular) portion of the ICA was seen to form a trifurcation at the limen insulae (Fig. 3A). The cavernous sinus was accessed and filled with cotton packing material (Fig. 3B).
FIG. 3.
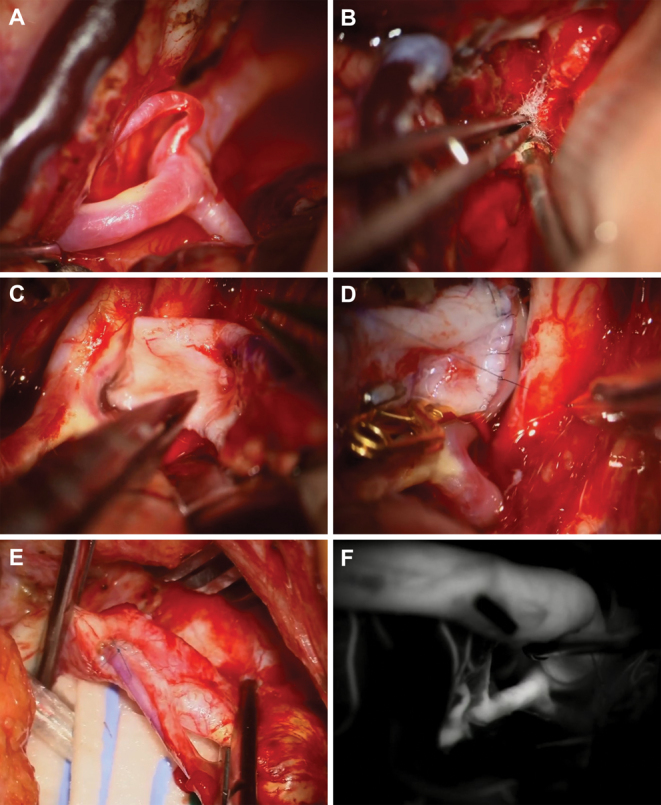
A: Splitting the sylvian fissure revealed the M2 segment of the ICA trifurcating at the limen insulae; the segment caliber was found to be too small for the graft. B:The cavernous sinus was accessed and filled with cotton packing material. C: Because the cephalic vein was severely mismatched to the M2 branch, the supraclinoid ICA was chosen as the new recipient. D: Anastomosis between the supraclinoid ICA and the cephalic vein was performed after trapping of the supraclinoid ICA distal to the ophthalmic artery and proximal to the choroidal artery, with an additional temporary clip on the posterior communicating artery. E: The CCA and the cephalic vein were sutured after temporary trapping of the CCA. F: Indocyanine green videoangiography confirmed patency of the cephalic vein graft bridging the supraclinoid ICA and CCA.
Next, the bypass was initiated. After harvest and preparation, the cephalic vein was found to be a severe mismatch for the M2 branch. As a result, the supraclinoid ICA was chosen as the new recipient (Fig. 3C). Temporary trapping of the supraclinoid ICA was conducted distal to the ophthalmic artery and proximal to the choroidal artery, with an additional temporary clip on the posterior communicating artery. First, the anastomosis between the supraclinoid ICA and the cephalic vein was performed so that the graft could be easily moved side to side to allow for continuous wall suturing (Fig. 3D). The graft was then tunneled with a chest tube to the neck. Temporary trapping of the CCA was performed to allow suturing of the CCA and the cephalic vein (Fig. 3E and Video 1).
VIDEO 1. Clip showing a CCA to supraclinoid ICA interpositional bypass and ICA sacrifice for an iatrogenic CCF. Used with permission from Barrow Neurological Institute, Phoenix, Arizona. Click here to view.
Patency was confirmed visually and with indocyanine green videoangiography (Fig. 3F). The cervical ICA was trapped with suture ligation in the neck, and a permanent clip was applied proximal to the ophthalmic artery intracranially. After completion of the procedure, postoperative catheter angiography was performed to confirm successful CCF treatment. The patient was then taken to the operating room on the following day to remove the balloon under direct endoscopic visualization, with closure using a nasoseptal flap.
The final graft construct can be described using the coding nomenclature proposed by Tayebi Meybodi et al.14 as L CCA (S-EC) CephV (E-SC) ICA (i.e., left CCA [side-to-endcontinuous] cephalic vein [end-to-sidecontinuous] ICA). The original surgical plan could be coded as ECA (S-E) RAG (S-E) M2 (i.e., external carotid artery [side-to-end] radial artery graft [side-to-end] M2 artery). However, during the operation, the patient was found to have a deep neck and high bifurcation, making external carotid artery exposure difficult. We considered taking down the digastric and stylomandibular ligament to mobilize the mandible but did not believe that doing so would yield sufficiently greater exposure. Although preoperative Doppler ultrasound findings indicated good flow, intraoperatively the radial artery was found to be occluded due to earlier endovascular procedures, leaving only 14 cm of viable graft. A graft longer than 21 cm was necessary for this procedure. Therefore, an alternative was sought. Rather than seeking an alternative anatomical site for grafting, the cephalic vein was selected as the graft of choice because of its substantial size and concurrent exposure during radial artery harvest. Because of earlier catheterization, the proximal cephalic vein was also thrombosed. However, the distal cephalic vein ran into the proximal basilic vein, and this “cephalobasilic” system was healthy and well-suited for use as a graft. The supraclinoid ICA was chosen as the recipient due to a severe mismatch between the M2 branch and the cephalic vein.
The patient tolerated the surgery well. Postoperatively, she had decreased vision and movement of the left eye but was otherwise neurologically intact. These symptoms are likely attributable to the CCF because the increased venous pressure within the cavernous sinus can affect the cranial nerves responsible for ocular motor function and vision. However, it is also possible that trapping of the ophthalmic artery during the bypass may have contributed to the patient's vision loss. She was discharged from the hospital to an acute rehabilitation facility. Postoperative angiography showed a patent bypass into the supraclinoid ICA; brisk perfusion of the left hemisphere, including the anterior cerebral artery (ACA), lenticulostriate arteries, and MCA; the stump of the ICA in the neck; and complete resolution of the fistula (Fig. 4).
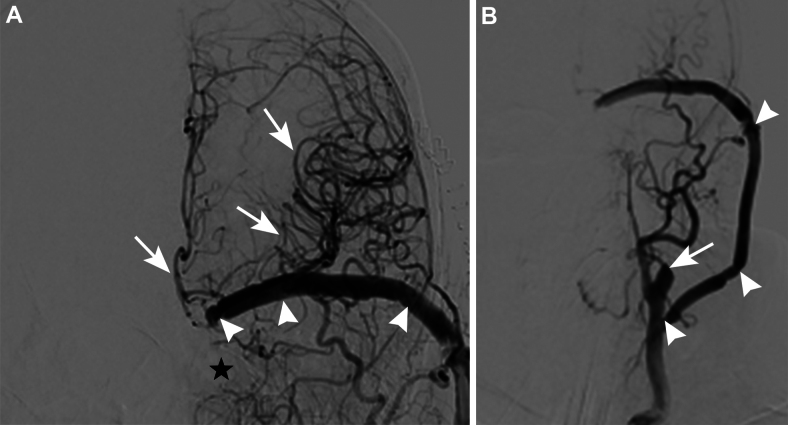
FIG. 4.
Postoperative cerebral angiography. A: Angiogram showing complete resolution of the fistula (black star). Arrowheads indicate patent bypass into the supraclinoid ICA. Arrows indicate brisk perfusion of the left hemisphere, including the ACA, lenticulostriate arteries, and MCA. B: Arrowheads indicate patent bypass into the supraclinoid ICA. The arrow indicates brisk perfusion of the stump of the ICA in the neck.
Literature Review
A PubMed search was conducted to identify relevant studies using a combination of keywords: “endoscopic,” “sinus surgery,” “iatrogenic,” “internal carotid artery injury,” “bypass,” and “radial artery graft.” We included articles describing iatrogenic ICA injury following endoscopic or microscopic sinus surgery, treated with extracranial-intracranial bypass. Although our case described a complication from FESS, cases describing iatrogenic ICA injury during FESS, EESBS, and microscopic sinus surgery were included in our literature review. Despite differences in scope, the management of ICA injuries, including the use of extracranial-intracranial bypass, is relevant to these procedures. In total, we found 6 articles reporting 13 cases in the literature, for a total of 14 cases, including our case (Table 1).
TABLE 1.
Literature review of endoscopic and microscopic iatrogenic ICA injury treated with extracranial-intracranial bypass
| Authors & Year, Case No. | Initial Diagnosis | Age (yrs)/Sex | Cause of Injury | Location of Injury | Angiography Findings | CR Procedure | Graft | Outcome |
| Rangel- Castilla et al., 201429 | ||||||||
| 1 | Pituitary adenoma | 29/F | Endoscopic transsphenoidal op | Cavernous ICA | PCF, large pseudoaneurysm | ICA-RAG-M2 | Radial artery | No neurological deficits |
| 2 | Pituitary adenoma | 45/M | Endoscopic transsphenoidal op | Cavernous ICA | PCF | ICA-RAG-M2 | Radial artery | No neurological deficits |
| 3 | Rathke cleft cyst | 38/M | Endoscopic transsphenoidal op | Cavernous ICA | PCF, large pseudoaneurysm | ICA-RAG-M2 | Radial artery | No neurological deficits |
| 4 | Recurrent skull base chordoma | 65/M | Endoscopic skull base tumor resection | Cavernous ICA | PCF, rapidly progressive dissection | ICA-RAG-M2 | Radial artery | No neurological deficits |
| Tantongtip et al., 201515 | ||||||||
| 5 | Pituitary adenoma | 78/F | Endoscopic transsphenoidal op | Cavernous ICA | Minor extravasation | ECA-RAG-ICA | Radial artery | Died 9 days postop |
| Mrak et al., 201831 | ||||||||
| 6 | Pituitary adenoma | 30/F | Endoscopic transsphenoidal op | Intracranial ICA | PCF | STA-MCA | NA | No neurological deficits |
| Usachev et al., 20214 | ||||||||
| 7 | Pituitary adenoma | 41/M | Endoscopic transsphenoidal op | Cavernous ICA | PCF, pseudoaneurysm | ICA-RAG-M2 & ICA occlusion w/ coil embolization | Radial artery | Visual field defect |
| 8 | Pituitary adenoma | 66/F | Endoscopic transsphenoidal op | Cavernous ICA | PCF | STA-MCA & ICA occlusion w/ coil embolization | NA | Hemiparesis |
| Hong et al., 20227 | ||||||||
| 9 | Pituitary adenoma | 71/M | Microscopic transsphenoidal op | Cavernous ICA | PCF, pseudoaneurysm | STA-MCA (double barrel) & endovascular trapping | NA | Dysarthria |
| 10 | Pituitary adenoma | 69/F | Microscopic transsphenoidal op | Cavernous ICA | PCF, pseudoaneurysm | STA-MCA (double barrel) & endovascular trapping | NA | No neurological deficits |
| 11 | Pituitary adenoma | 66/M | Microscopic transsphenoidal op | Cavernous ICA | PCF | STA-RAG-MCA | Radial artery | Dysarthria, hemiparesis |
| 12 | Nasopharyngeal cancer | 37/M | Endoscopic transsphenoidal op | Cavernous ICA | Sufficient collateral flow | STA-MCA & endovascular trapping | NA | Dysarthria, hemiparesis |
| Teramoto et al., 202230 | ||||||||
| 13 | Pituitary adenoma | 65/M | Endoscopic transsphenoidal op | Petrous ICA | CCF | STA-MCA double bypass & ICA occlusion w/ coil embolization | NA | No neurological deficits |
| Present study | ||||||||
| 14 | Nasal polyp | 58/F | Endoscopic sinus op | Cavernous ICA | CCF | CCA-CephV-ICA | Cephalic vein | Visual field defect |
CephV = cephalic vein; CR = cerebral revascularization; ECA = external carotid artery; NA = not applicable; PCF = poor collateral flow; RAG = radial artery graft; STA = superficial temporal artery.
Of these cases, 8 used grafts for revascularization, with 7 using the radial artery and 1 using the cephalic vein (our case). The remaining 6 bypass procedures involved anastomosing the superficial temporal artery and the MCA. All bypass procedures were successful except for the case reported by Tantongtip et al.,15 in which the patient died 9 days after the operation. Seven patients (50%) were treated without long-term neurological deficits. In the remaining cases, long-term neurological deficits included a visual field defect, dysarthria, hemiparesis, or a combination of these symptoms. It is worth noting that initial attempts at endovascular treatment were unsuccessful in 2 cases (cases 8 and 12), resulting in the subsequent onset of neurological symptoms. After successful bypass procedures, initial symptoms persisted, but new symptoms did not develop.
Patient Informed Consent
The necessary patient informed consent was obtained in this study.
Discussion
Observations
Endoscopic sinus surgery requires a thorough understanding of the nasal and paranasal anatomy. FESS addresses anatomical issues within the paranasal sinuses, specifically targeting the ostiomeatal complex, maxillary sinuses, ethmoid sinuses, frontal sinuses, and sphenoid sinuses to restore normal drainage and ventilation pathways.16 EESBS has become the standard approach for addressing skull base lesions extending from the frontal sinus to the second cervical vertebra sagittally and from the roof of the orbit to the floor of the middle cranial fossa coronally.17 Endoscopic sinus surgery has gained popularity because it offers several advantages, including avoiding cutaneous incisions, being minimally invasive, and requiring a shorter postoperative hospital stay than other techniques.18 Potential complications of endoscopic sinus surgery include hemorrhage, rhinorrhea, and infection. Among these potential complications, ICA injury is the most serious and carries the highest risk of mortality.19 The ICA cavernous segment is most prone to injury in transnasal approaches, and such injury results in overwhelming hemorrhage, with the potential concurrent formation of a direct high-flow CCF (Barrow type A).13, 20
The goal of CCF treatment is to achieve complete occlusion of the fistula while simultaneously preserving normal blood flow through the ICA. Although endovascular embolization of the fistula is often the primary consideration for treatment, there are several potential complications of this approach to consider. For example, excessive packing of coiling material into the cavernous sinus can worsen visual symptoms.21 Even with balloon test occlusion confirmation of ischemic tolerance in response to ICA occlusion, ICA sacrifice without distal revascularization can lead to chronic hemodynamic stress.22 Although covered stents or flow diverters can initially yield favorable results in preserving the ICA, the risk of delayed thrombosis with current stenting methods remains a concern.23
Although high-flow bypass procedures are complex and technically challenging, several studies have confirmed their overall safety, long-term success in graft patency, and low rates of associated morbidity and mortality.24–28 In the absence of established treatment guidelines for managing iatrogenic ICA injury, determining whether a high-flow bypass is indicated remains difficult. A treatment algorithm proposed by Usachev et al.4 suggests that high-flow bypass is indicated when reconstruction is not possible and angiography shows the absence of collateral circulation. In our review of the literature, a total of 6 studies and 13 cases were identified in which endoscopic or microscopic sinus surgery resulted in iatrogenic ICA injury that was treated with extracranial-intracranial bypass.4, 7, 15, 29–31 In 8 cases, including our case, grafts were used for revascularization, with the radial artery used in 7 cases and the cephalic vein used in our case. All but one of the bypass procedures were successful. Of 14 patients, 7 (50%) had successful treatment without long-term neurological deficits, and 7 (50%) had various long-term neurological deficits, including visual field defects, dysarthria, hemiparesis, or a combination of these symptoms. Our literature review revealed that, in the majority of cases, extracranial-intracranial bypass surgery for iatrogenic endoscopic or microscopic ICA injury resulted in relatively favorable outcomes. However, it is important to note that unfavorable outcomes may be underreported.
Several treatment options were considered but ultimately not chosen for our patient. A low-flow bypass procedure, such as a left superficial temporal artery side-to-end M4 MCA bypass with endovascular sacrifice, was believed to be inappropriate due to concerns that there would be insufficient blood flow through the superficial temporal artery. In addition, there was a concern that the accuracy of balloon test occlusion results might be compromised due to the deep sedation of the patient that would be required to maintain Foley balloon placement. Endovascular intra-arterial reconstruction with flow diversion was considered a less viable option due to continued bleeding and the persistence of the fistula with balloon takedown.
For extracranial-intracranial bypass surgery, the radial artery and saphenous vein are the traditional grafts of choice. The radial artery is generally preferred because it is associated with superior long-term patency rates.32 The radial artery is a natural physiological conduit for arterial blood and has a diameter (mean [standard deviation] diameter 3.55 [0.45] mm) that is compatible with that of the M2 artery, resulting in smoother laminar blood flow and reduced turbulence. Turbulent blood flow can lead to delayed graft occlusion.33 In addition, arterial grafts are more resistant to kinking or twisting than vein grafts, and their lack of valves ensures that they remain open even at lower flow rates.33 However, in situations in which a viable radial artery is not available, there has been limited exploration of the cephalic vein as an alternative conduit for cerebral bypass. In 2016, Nossek et al.34 published the first article discussing the use of the cephalic vein for cerebral bypass procedures, demonstrating its viability when the radial artery or saphenous vein are not feasible options. In our case, the radial artery was found to be occluded because of earlier endovascular procedures. The cephalic vein was selected as the graft of choice because of its substantial size, concurrent exposure during radial artery harvest, and minimal additional risk. The use of the cephalic vein graft effectively resolved the fistula and resulted in a successful surgical outcome.
Lessons
In cases involving ICA injury and CCF formation, microsurgical trapping with high-flow bypass is a favorable treatment option because it allows for full augmentation of cerebral perfusion, provides definitive treatment of CCFs by removing all recruitable ICA skull base feeders, allows for direct cavernous sinus packing, preserves the ophthalmic artery for maximal vision recovery, and forgoes the antiplatelet loading needed with covered stents in the setting of a high risk of hemorrhage. Moreover, our experience suggests that the cephalic vein is a viable graft option to consider when unexpected challenges arise with a radial artery graft. The cephalic vein offers the advantage of being harvested from the same incision site without introducing additional morbidity.
Acknowledgments
We thank the staff of Neuroscience Publications at Barrow Neurological Institute for assistance with manuscript preparation.
Disclosures
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Author Contributions
Conception and design: Lawton, Rahmani. Acquisition of data: Lawton, Rahmani, Benet, Kim, Scherschinski, Catapano, Anthony, Jadhav, Ducruet, Little, Santarelli, Stevens, Jategaonkar. Analysis and interpretation of data: Lawton, Wehbi, Rahmani, Benet, Kim, Catapano, Anthony, Jadhav, Ducruet, Little, Santarelli, Stevens, Jategaonkar. Drafting the article: Lawton, Wehbi, Rahmani, Little, Jategaonkar. Critically revising the article: Lawton, Wehbi, Rahmani, Benet, Kim, Scherschinski, Catapano, Anthony, Jadhav, Ducruet, Little, Santarelli, Stevens, Jategaonkar. Reviewed submitted version of manuscript: Lawton, Rahmani, Benet, Kim, Scherschinski, Catapano, Anthony, Jadhav, Ducruet, Albuquerque, Little, Santarelli, Stevens, Jategaonkar. Approved the final version of the manuscript on behalf of all authors: Lawton. Study supervision: Lawton.
Supplemental Information
Videos
Video 1. https://vimeo.com/995052697.
Correspondence
Michael T. Lawton: c/o Neuroscience Publications, Barrow Neurological Institute, St. Joseph’s Hospital and Medical Center, Phoenix, AZ. neuropub@barrowneuro.org.
References
- 1.Zhang Y, Tian Z, Li C, et al. A modified endovascular treatment protocol for iatrogenic internal carotid artery injuries following endoscopic endonasal surgery. J Neurosurg. 2019;132(2):343-350. [DOI] [PubMed] [Google Scholar]
- 2.Valentine R, Wormald PJ. Controlling the surgical field during a large endoscopic vascular injury. Laryngoscope. 2011;121(3):562-566. [DOI] [PubMed] [Google Scholar]
- 3.Padhye V, Valentine R, Paramasivan S, et al. Early and late complications of endoscopic hemostatic techniques following different carotid artery injury characteristics. Int Forum Allergy Rhinol. 2014;4(8):651-657. [DOI] [PubMed] [Google Scholar]
- 4.Usachev D, Sharipov O, Abdali A, et al. Internal carotid artery injury in transsphenoidal surgery: tenets for its avoidance and refit—a clinical study. Brain Sci. 2021;11(1):99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weidenbecher M, Huk WJ, Iro H. Internal carotid artery injury during functional endoscopic sinus surgery and its management. Eur Arch Otorhinolaryngol. 2005;262(8):640-645. [DOI] [PubMed] [Google Scholar]
- 6.Dalziel K, Stein K, Round A, Garside R, Royle P. Endoscopic sinus surgery for the excision of nasal polyps: a systematic review of safety and effectiveness. Am J Rhinol. 2006;20(5):506-519. [DOI] [PubMed] [Google Scholar]
- 7.Hong CK, Byun J, Park W, et al. Management of internal carotid artery injury during transsphenoidal surgery: a case series and suggestion for optimal management. World Neurosurg. 2022;163:e230-e237. [DOI] [PubMed] [Google Scholar]
- 8.Muto J, Carrau RL, Oyama K, Otto BA, Prevedello DM. Training model for control of an internal carotid artery injury during transsphenoidal surgery. Laryngoscope. 2017;127(1):38-43. [DOI] [PubMed] [Google Scholar]
- 9.Kohli GS, Patel BC. Carotid cavernous fistula. In: StatPearls. [Internet] StatPearls; Publishing;2024. [PubMed] [Google Scholar]
- 10.Sheinberg DL, Brunet MC, Chen SH, Luther E, Starke RM. Iatrogenic direct carotid-cavernous fistula following mechanical thrombectomy: a case report and review of the literature. Cureus. 2020;12(4):e7524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gardner PA, Snyderman CH, Fernandez-Miranda JC, Jankowitz BT. Management of major vascular injury during endoscopic endonasal skull base surgery. Otolaryngol Clin North Am. 2016;49(3):819-828. [DOI] [PubMed] [Google Scholar]
- 12.Pacca P, Jhawar SS, Seclen DV, et al. “Live Cadaver” model for internal carotid artery injury simulation in endoscopic endonasal skull base surgery. Oper Neurosurg (Hagerstown). 2017;13(6):732-738. [DOI] [PubMed] [Google Scholar]
- 13.Matoušek P, Krejčí T, Misiorzová E, et al. Internal carotid injury during skull base surgery—case report and a review of the literature. Brain Sci. 2022;12(9):1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tayebi Meybodi A, Gadhiya A, Borba Moreira L, Lawton MT. Coding cerebral bypasses: a proposed nomenclature to better describe bypass constructs and revascularization techniques. J Neurosurg. 2021;136(1):163-174. [DOI] [PubMed] [Google Scholar]
- 15.Tantongtip D, Fratianni A, Jenkner J, Arnold S, Spetzger U. Surgical treatment of inadvertent internal carotid artery lesion by extraintracranial high-flow bypass. A case report and review of the literature. J Neurol Surg Rep. 2015;76(1):e100-e104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Homsi MT, Gaffey MM. Sinus endoscopic surgery. In: StatPearls. [Internet] StatPearls; Publishing; 2024. [PubMed] [Google Scholar]
- 17.Kahilogullari G, Bahadır B, Bozkurt M, Akcalar S, Balci S, Arat A. Carotid artery-cavernous segment injury during an endoscopic endonasal surgery: a case report and literature review of the overlooked option for surgical trapping in the hyperacute phase. J Neurol Surg Rep. 2021;82(4):e53-e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Snyderman CH, Pant H, Carrau RL, Prevedello D, Gardner P, Kassam AB. What are the limits of endoscopic sinus surgery?: the expanded endonasal approach to the skull base. Keio J Med. 2009;58(3):152-160. [DOI] [PubMed] [Google Scholar]
- 19.Gardner PA, Tormenti MJ, Pant H, Fernandez-Miranda JC, Snyderman CH, Horowitz MB. Carotid artery injury during endoscopic endonasal skull base surgery: incidence and outcomes. Neurosurgery. 2013;73(2suppl Operative):269-270. [DOI] [PubMed] [Google Scholar]
- 20.Valentine R, Wormald PJ. Carotid artery injury after endonasal surgery. Otolaryngol Clin North Am. 2011;44(5):1059-1079. [DOI] [PubMed] [Google Scholar]
- 21.Hasegawa H, Inoue T, Tamura A, Saito I. Urgent treatment of severe symptomatic direct carotid cavernous fistula caused by ruptured cavernous internal carotid artery aneurysm using high-flow bypass, proximal ligation, and direct distal clipping: technical case report. Surg Neurol Int. 2014;5:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niiro M, Shimozuru T, Nakamura K, Kadota K, Kuratsu J. Long-term follow-up study of patients with cavernous sinus aneurysm treated by proximal occlusion. Neurol Med Chir (Tokyo). 2000;40(2):88-97. [DOI] [PubMed] [Google Scholar]
- 23.Ellis JA, Goldstein H, Connolly ES, Jr, Meyers PM. Carotid-cavernous fistulas. Neurosurg Focus. 2012;32(5):E9. [DOI] [PubMed] [Google Scholar]
- 24.Houkin K, Kamiyama H, Kuroda S, Ishikawa T, Takahashi A, Abe H. Long-term patency of radial artery graft bypass for reconstruction of the internal carotid artery. Technical note. J Neurosurg. 1999;90(4):786-790. [DOI] [PubMed] [Google Scholar]
- 25.Ishishita Y, Tanikawa R, Noda K, et al. Universal extracranial-intracranial graft bypass for large or giant internal carotid aneurysms: techniques and results in 38 consecutive patients. World Neurosurg. 2014;82(1-2):130-139. [DOI] [PubMed] [Google Scholar]
- 26.Kalani MY, Zabramski JM, Hu YC, Spetzler RF. Extracranial-intracranial bypass and vessel occlusion for the treatment of unclippable giant middle cerebral artery aneurysms. Neurosurgery. 2013;72(3):428-436. [DOI] [PubMed] [Google Scholar]
- 27.Sekhar LN, Bucur SD, Bank WO, Wright DC. Venous and arterial bypass grafts for difficult tumors, aneurysms, and occlusive vascular lesions: evolution of surgical treatment and improved graft results. Neurosurgery. 1999;44(6):1207-1224. [DOI] [PubMed] [Google Scholar]
- 28.Sughrue ME, Saloner D, Rayz VL, Lawton MT. Giant intracranial aneurysms: evolution of management in a contemporary surgical series. Neurosurgery. 2011;69(6):1261-1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rangel-Castilla L, McDougall CG, Spetzler RF, Nakaji P. Urgent cerebral revascularization bypass surgery for iatrogenic skull base internal carotid artery injury. Neurosurgery. 2014;10(Suppl 4):640-648. [DOI] [PubMed] [Google Scholar]
- 30.Teramoto S, Tahara S, Murai Y, et al. Injury to the extrasellar portion of the internal carotid artery during endoscopic transsphenoidal surgery: a case report. Front Surg. 2022;9:895233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mrak G, Nemir J, Brgic K, Baric H, Paladino J, Stambolija V. Cerebral bypass surgery for internal carotid artery occlusion, complex supraclinoid carotid artery aneurysm, and tumors: a report of four cases. Asian J Neurosurg. 2018;13(3):938-942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gaudino M, Benedetto U, Fremes S, et al. Radial-artery or saphenous-vein grafts in coronary-artery bypass surgery. N Engl J Med. 2018;378(22):2069-2077. [DOI] [PubMed] [Google Scholar]
- 33.Roh SW, Ahn JS, Sung HY, Jung YJ, Kwun BD, Kim CJ. Extracranial-intracranial bypass surgery using a radial artery interposition graft for cerebrovascular diseases. J Korean Neurosurg Soc. 2011;50(3):185-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nossek E, Costantino PD, Chalif DJ, Ortiz RA, Dehdashti AR, Langer DJ. Forearm cephalic vein graft for short, “middle”-flow, internal maxillary artery to middle cerebral artery bypass. Oper Neurosurg (Hagerstown). 2016;12(2):99-105. [DOI] [PubMed] [Google Scholar]