Abstract
Aim
Despite the emphasis on sensory dysfunction phenotypes in the revised diagnostic criteria for autism spectrum disorder (ASD), there has been limited research, particularly in the field of neurobiology, investigating the concordance in sensory features between individuals with ASD and their genetic relatives. Therefore, our objective was to examine whether neurobehavioral sensory patterns could serve as endophenotypic markers for ASD.
Methods
We combined questionnaire‐ and lab‐based sensory evaluations with sensory fMRI measures to examine the patterns of sensory responsivity in 30 clinically diagnosed with ASD, 26 matched controls (CON), and 48 biological parents for both groups (27 parents of individuals with ASD [P‐ASD] and 21 for individuals with CON [P‐CON]).
Results
The ASD and P‐ASD groups had higher sensory responsivity and rated sensory stimuli as more unpleasant than the CON and P‐CON groups, respectively. They also exhibited greater hemodynamic responses within the sensory cortices. Overlapping activations were observed within these sensory cortices in the ASD and P‐ASD groups. Using a machine learning approach with robust prediction models across cohorts, we demonstrated that the sensory profile of biological parents accurately predicted the likelihood of their offspring having ASD, achieving a prediction accuracy of 71.4%.
Conclusions
These findings provide support for the hereditary basis of sensory alterations in ASD and suggest a potential avenue to improve ASD diagnosis by utilizing the sensory signature of biological parents, especially in families with a high risk of ASD. This approach holds promising prospects for early detection, even before the birth of the offspring.
Keywords: autism spectrum disorder, endophenotype, machine learning, parent–child dyads, sensory responsivity
The early detection of autism spectrum disorder (ASD) is widely recognized as a critical factor in promoting early intervention and improving outcomes, but it remains a challenge. 1 Nonetheless, recent research using magnetic resonance imaging (MRI) and machine learning techniques in infants at high familial risk for ASD reveals that early postnatal changes in brain imaging could aid in the ASD diagnosis. 2 Consequently, by combining heritable profiles for sensory processing abnormalities 3 associated with ASD candidate genes 4 , 5 , 6 with neuroimaging of the underlying neurocognitive processes in parents at high familial risk for ASD, we can further glean more precisely the underlying genetics, enabling us to identify the predicting factors for ASD in their offspring and potentially even make earlier diagnosis of before birth. 7
ASD is one of the most common neurodevelopmental disorders, with genetic factors accounting for up to 64%–91% of the risk. 8 , 9 Previous searches for the autistic endophenotype mostly revealed a wide range of social and behavioral phenotypes shared across genetic relatives of individuals with ASD. 10 , 11 , 12 , 13 , 14 However, endophenotype studies on other critical ASD‐related traits, such as atypical sensory processing, appear to be largely neglected.
Unusual responses to sensory stimuli have been observed in individuals with ASD since its earliest descriptions. 15 , 16 This behavioral trait is estimated to occur in up to 90% of ASD individuals 17 and has been incorporated into the diagnostic criteria. 18 Genetic research suggests that parents with high genetic liability for ASD exhibit more atypical sensory processing than those with low or no ASD genetic liability. 19 Additionally, non‐affected family members of individuals with ASD share some aspects related to sensory symptomatology with their affected relatives. 20 , 21 , 22 , 23 However, no study has proposed a predictive model of ASD based on sensory profiles, and we still lack a robust neurobehavioral sensory signature capable of identifying the risk of having offspring with ASD in non‐affected parents. Developing such a sensory signature would advance our understanding of sensory processing and have significant clinical implications.
In the present study, we examined whether the patterns of sensory responsivity at both behavioral and neural levels could serve as endophenotypic markers for ASD. We studied the behavioral and neural sensory profiles in individuals with clinically ascertained ASD and their unaffected biological parents (P‐ASD) as well as matched controls (CON) and their parents (P‐CON). We hypothesized that, compared to the control group, individuals with ASD and their parents would show higher levels of sensory responsiveness in behavioral tasks, accompanied by increased recruitment of sensory processing brain regions. Furthermore, we predicted that the sensory signatures in the parent–child dyads would be predictive within high‐risk families that shared underlying genetics of heritable abnormalities in sensory processing.
Methods
Participants
A total of 113 participants were initially recruited for the study. However, due to serious motion artifacts, nine individuals (consisting of four ASD, two CON, one P‐ASD, and two P‐CON) were excluded for further analyses. At the end, the current study included 104 participants, with 30 individuals with ASD and 26 matched controls (CON) for the offspring group and 27 P‐ASD and 21 P‐CON for the parent group. Participants with ASD (aged 12 and up) must have previously been diagnosed with ASD based on the DSM‐5 diagnostic criteria 18 and have an ASD severity level in the DSM‐5 classification ranging from mild (level 1) to moderate (level 2) by certified and experienced physicians. All participants had a full‐scale intelligence quotient (FSIQ) above 80, as assessed by the Wechsler Abbreviated Scale of Intelligence (WASI). 24 Each participant's hand dominance was confirmed with the Edinburgh Handedness Inventory. 25 Exclusion criteria for the ASD group include comorbid psychiatric or medical conditions, a history of head injury, or a genetic disorder associated with ASD. Participants were excluded from the P‐CON group if any of their biological children had a developmental disorder or if there were suspicions that their child might have ASD. Participants with CON and their parents were recruited from local schools and communities. All CON and P‐CON participants were screened for traits and behaviors of ASD using age‐specific versions of the Autism‐Spectrum Quotient (AQ), and for major psychiatric illnesses by conducting structured interviews. For adolescents aged 12–15 years, the AQ‐Adolescent was used, 26 while for participants aged 16 years and older, the AQ‐Adult was used. 27 There were no significant differences in age, years of education, sex, handedness, and the FSIQ scores between ASD and CON and between P‐ASD and P‐CON (Table 1).
Table 1.
Demographic and clinical variables of the study participants
| ASD (n = 30) | CON (n = 26) | P‐ASD (n = 27) | P‐CON (n = 21) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | P | Cohen's d | Mean | SD | Mean | SD | P | Cohen's d | |
| Age | 18.77 | 6.09 | 18.96 | 5.13 | 0.89 | 0.03 | 49.07 | 6.03 | 46.62 | 5.66 | 0.16 | 0.41 |
| Education | 11.57 | 3.88 | 12.62 | 4.08 | 0.33 | 0.26 | 15.70 | 2.67 | 15.86 | 3.48 | 0.86 | 0.05 |
| Sex (F/M) | 4/26 | 5/21 | 0.55 | — | 21/6 | 17/4 | 0.79 | — | ||||
| Handedness (R/L) | 26/4 | 23/3 | 0.84 | — | 27/0 | 21/0 | — | — | ||||
| The FSIQ | 108.37 | 9.56 | 111.31 | 5.76 | 0.18 | 0.37 | 109.58 | 4.92 | 110.81 | 4.42 | 0.40 | 0.26 |
| The AASP | ||||||||||||
| Taste/Smell | 20.33 | 5.15 | 20.08 | 3.02 | 0.83 | 0.06 | 20.81 | 3.73 | 18.86 | 3.01 | 0.06 | 0.58 |
| Movement | 18.80 | 4.01 | 20.00 | 3.37 | 0.24 | 0.32 | 19.19 | 3.71 | 18.48 | 3.17 | 0.49 | 0.21 |
| Visual | 25.23 | 6.10 | 22.73 | 3.38 | 0.07 | 0.51 | 26.19 | 4.13 | 23.14 | 2.71 | <0.01* | 0.87 |
| Tactile | 33.77 | 8.29 | 29.81 | 4.17 | 0.03* | 0.60 | 31.85 | 5.43 | 27.90 | 5.36 | 0.02* | 0.63 |
| Activity level | 32.40 | 5.14 | 28.00 | 3.84 | <0.01* | 0.97 | 27.48 | 4.64 | 24.76 | 2.79 | 0.02* | 0.73 |
| Auditory | 34.47 | 5.39 | 27.92 | 5.15 | <0.01* | 1.24 | 29.85 | 5.61 | 26.81 | 4.30 | 0.04* | 0.61 |
| Total scores | 165.00 | 24.59 | 148.54 | 14.23 | <0.01* | 0.82 | 155.37 | 20.06 | 139.90 | 13.62 | <0.01* | 0.90 |
| The SORI | 16.42 | 8.61 | 11.08 | 6.72 | 0.02* | 0.69 | 13.00 | 12.05 | 8.71 | 5.75 | 0.14* | 0.45 |
| The AQ | ||||||||||||
| Social skills | 6.04 | 2.89 | 3.08 | 2.50 | <0.01* | 1.10 | 3.85 | 2.44 | 3.38 | 1.86 | 0.48 | 0.22 |
| Attentional switch | 6.74 | 1.51 | 4.88 | 1.56 | <0.01* | 1.21 | 4.54 | 1.79 | 3.62 | 1.77 | 0.09 | 0.52 |
| Attention to detail | 5.59 | 2.50 | 3.68 | 1.99 | <0.01* | 0.85 | 4.96 | 2.07 | 3.29 | 2.15 | <0.01* | 0.79 |
| Communication | 5.78 | 2.49 | 2.56 | 1.81 | <0.01* | 1.48 | 2.73 | 1.69 | 2.48 | 1.72 | 0.61 | 0.15 |
| Imagination | 4.81 | 2.22 | 2.92 | 1.63 | <0.01* | 0.97 | 3.54 | 1.45 | 3.10 | 1.34 | 0.29 | 0.32 |
| Total scores | 28.96 | 7.56 | 17.16 | 4.43 | <0.01* | 1.90 | 19.62 | 5.37 | 15.95 | 2.31 | <0.01* | 0.89 |
| The TAS‐20 | ||||||||||||
| Identifying emotions | 16.23 | 3.88 | 11.90 | 3.10 | <0.01* | 1.23 | 13.85 | 4.29 | 11.78 | 2.13 | 0.07 | 0.61 |
| Describing emotions | 20.50 | 5.49 | 15.62 | 3.37 | <0.01* | 1.07 | 17.07 | 4.71 | 13.78 | 3.61 | 0.02* | 0.78 |
| External‐oriented | 21.50 | 4.73 | 19.05 | 4.96 | 0.08 | 0.56 | 20.11 | 3.26 | 20.56 | 3.99 | 0.68 | 0.12 |
| Total scores | 58.23 | 10.61 | 46.57 | 8.68 | <0.01* | 1.20 | 51.04 | 10.37 | 46.11 | 6.74 | 0.08 | 0.56 |
| The RBS‐R | ||||||||||||
| Stereotypic | 4.62 | 4.22 | 1.50 | 2.25 | <0.01* | 0.92 | 1.22 | 2.50 | 0.14 | 0.48 | 0.06 | 0.59 |
| Self‐injury | 3.00 | 4.26 | 0.88 | 1.73 | 0.02* | 0.65 | 0.44 | 1.09 | 0.14 | 0.66 | 0.27 | 0.33 |
| Compulsive | 6.62 | 5.44 | 2.12 | 3.05 | <0.01* | 1.02 | 2.33 | 3.44 | 1.71 | 2.19 | 0.48 | 0.22 |
| Ritualistic | 3.46 | 2.75 | 1.50 | 2.47 | <0.01* | 0.75 | 1.81 | 3.52 | 0.19 | 0.60 | 0.04* | 0.64 |
| Sameness | 8.50 | 5.78 | 1.35 | 1.85 | <0.01* | 1.67 | 3.41 | 6.01 | 0.90 | 2.07 | 0.08 | 0.56 |
| Restricted | 4.73 | 2.97 | 1.23 | 1.93 | <0.01* | 1.40 | 1.48 | 2.98 | 0.43 | 0.98 | 0.13 | 0.47 |
| Total scores | 30.92 | 19.83 | 8.58 | 9.46 | <0.01* | 1.44 | 10.70 | 15.99 | 3.52 | 5.39 | 0.06 | 0.60 |
Abbreviations: AASP, Adolescent/Adult Sensory Profile; AQ, Autism Spectrum Quotient; ASD, autism spectrum condition; CON, matched controls; F, Females; FSIQ, Full Scale Intelligence Quotient; L, Left; M, Males; P‐ASD, parents of ASD; P‐CON, parents of the CON; R, Right; RBS‐R, Repetitive Behaviors Scale‐Revised; SORI, Sensory Over‐Responsivity Inventory; TAS‐20, Toronto Alexithymia Scale‐20.
P‐value <0.05.
All participants had a normal corrected vision and bilateral peripheral hearing during testing. The demographic characteristics of the four groups of participants are summarized in Table 1. Informed assent and consent were acquired from all participants and their parents. All procedures in the present study were approved by the Institutional Review Board of National Chiao Tung University (IRB number: NCTU‐REC‐106‐053) and conducted in accordance with the Declaration of Helsinki.
Procedures
Before fMRI scanning, each participant underwent a series of the questionnaire‐ and lab‐based evaluations individually. The self‐report questionnaires include an information sheet, consent form, the Adolescent/Adult Sensory Profile (AASP) 28 , 29 the Sensory Over‐Responsivity Inventory (SORI) 30 the AQ, 26 , 27 the Repetitive Behavior Scale‐Revised (RBS‐R) 31 and the Toronto Alexithymia Scale (TAS‐20). 32 , 33 Please refer to the Supplementary Materials for details. The WASI was administrated by a licensed psychologist. In addition, the lab‐based sensory evaluations were performed in a quiet evaluation room, and all participants were asked to rate the unpleasantness of each stimulus (Supplementary Materials and Fig. S1).
MRI data acquisition, image processing, and analysis
Structural and functional MRI data were acquired on a 3 T MRI scanner (Skyra; Siemens, Erlangen, Germany) with a high‐resolution 20‐channel head array coil. Image processing and analysis were carried out using SPM8 (Wellcome Department of Imaging Neuroscience, London, UK). Participants took part in a single fMRI session in which neural responses to visual, auditory, and tactile stimuli were measured in three separate runs (Fig. S2). Please refer to Supplementary Materials for details.
We performed ROI analyses to further prod into the activation patterns within the brain regions previously reported in studies concerning sensory processing in ASD. Activities in the specific regions of interest (ROIs) were analyzed, including the primary visual cortex (x 0, y − 82, z − 2), the superior temporal gyrus (STG; −46, −14, 4), the primary somatosensory cortex (SI/SII; −52, −22, 24), the insula (−34, −16, 8), and the superior frontal gyrus (SFG, 4, 22, 46). Data extraction for the ROI analyses was performed using the MarsBaR toolbox (http://marsbar.sourceforge.net/) implemented in SPM8. The ROIs were defined as a 5‐mm spherical region centered on the coordinates determined on recent fMRI sensory studies in ASD and using similar sensory stimuli. 34 , 35 The individual mean parameter estimates (beta values) were then subject to an ANOVA for repeated measures to test for the main effects of the group, levels of each sensory stimulus, and their interactions.
Statistical analysis
The chi‐square test for categorical variables and independent t‐test for continuous variables were used to compare the demographic characteristics and questionnaire results of the status groups (ASD vs. CON) and parent groups (P‐ASD vs. P‐CON). A two‐way mixed‐design analysis of variance (ANOVA) was utilized to identify differences in performance on the lab‐based sensory evaluations between the ASD and CON and between the P‐ASD and P‐CON. Then, we performed intraclass correlation coefficients to quantify the agreement between parent–child pairs and establish consistency between the outcome measurements for the pairs (a biologically full parent–child pair). In addition, permutation tests were conducted to examine the statistical significance of the observed correlation patterns. A permuted dataset was generated at each iteration by shuffling the new parent–child pairs (each child was now paired with a non‐biological father or mother) while keeping the pair information. To construct a null distribution, we repeated this procedure 5000 times. Further linear regression analyses were conducted to examine whether the sensory features of participants may predict the levels of autistic traits within each group. The probability for entry in stepwise regression was set at 0.05. Adjusted R 2 value, standardized coefficient (β), and incremental R 2 were provided to better estimate the contribution for each predictor in the model.
Machine‐learning analysis
Development of an Artificial Neural Network (ANN) structure of sensory profile for AQ score prediction
Given the innate capability of deep learning ANN to predict continuous variables, we used this method to predict individuals' autistic traits. While several researchers have used the backpropagation (BP) algorithm in the areas of biomechanics and neuroimaging, this study develops a feedforward Back‐Propagation Artificial Neural Network (BP‐ANN) consisting of three layers of nodes: two hidden layers and one output layer, with one node in the case of the autistic quotient score (AQ). The number of nodes in the two hidden layers was optimally decided by minimizing the mean square (MSE) output on a training set. The mean square error (MSE) is minimized during the training process. Each subject in the training and test populations is represented by a v = 16 sensory profile feature vector that includes AASP, fMRI blood‐oxygen‐level dependent (BOLD) response, and unpleasantness ratings induced by the sensory tasks (Supplementary Materials).
Development of a LibSVM Classifier for the prediction of ASD diagnosis
Considering that ASD diagnosis essentially involves a binary classification (either diagnosed or not), the LIbSVM Classifier, inherently built for binary classification, emerges as a suitable choice for this purpose. In a second machine‐learning analysis, we used established techniques for multivariate pattern classification to identify the predicted diagnostic value of ASD using the combined behavioral and fMRI sensory profiles. We investigated whether the sensory profile shows diagnostic sensitivity for ASD using linear classifiers implemented by the LIBSVM toolbox 36 and whether the diagnostic prediction of the sensory profile model could be generalized from unaffected biological parents to the offspring. The default parameters of the linear SVM (C = 1) were applied (Supplementary Materials).
Results
Behavioral aspects of the sensory profile
Questionnaire‐based assessments
ASD vs. CON. There were significant differences between the two child groups on most of the Adolescent/Adult Sensory Profile (AASP) scores, with participants with ASD, relative to those in the CON, scoring higher on the tactile scores, activity level scores, auditory scores, and total scores of the AASP. In addition, the Sensory Over‐Responsivity Inventory (SORI) scores for the ASD group were also significantly higher than the CON group. These findings suggest that children with ASD exhibit hyper‐responsivity to sensory stimuli, which is consistent with previous research on sensory features in ASD. As expected, the ASD group scored higher than the CON group on all subscale scores and total scores of the Autism Spectrum Quotient (AQ), on the total scores of the Toronto Alexithymia Scale‐20 (TAS‐20), and subscale scores and total scores of the Repetitive Behavior Scale‐Revised (RBS‐R) (Table 1 and Fig. 1). Regarding the correlations of ASD symptomatology, both the total scores of the AASP and the total scores of the SORI was found to positively correlate with the communication scores of the AQ (AASP: r = 0.44, P = 0.02; SORI: r = 0.52, P < 0.01) in participants with ASD, in addition to a positive correlation between the total scores of the SORI and the total scores of the RBS‐R (r = 0.53, P < 0.01). In contrast, no significant correlations between sensory responsiveness and ASD‐related symptomatology were found in the CON group (all P >0.05).
Fig. 1.
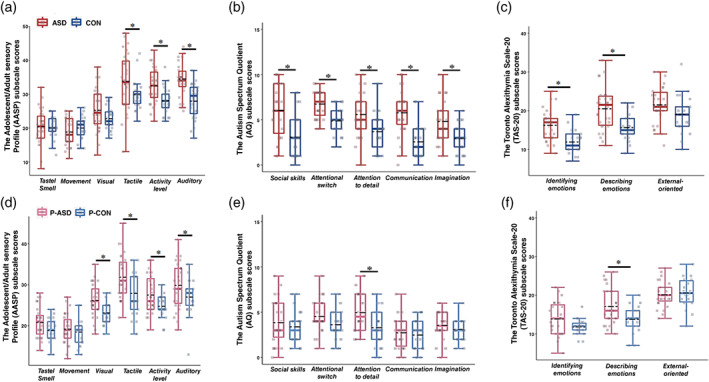
Comparisons of the AASP, the AQ, and the TAS‐20 subscales between children groups (ASD vs. CON) (a–c) and between parents groups (P‐ASD vs. P‐CON) (d–f). Boxes represent the 25th to the 75th centiles, and the black dotted and colored solid lines inside the box indicate the mean and median of the data set, respectively. *P < 0.05.
P‐ASD vs. P‐CON. Parent groups differed significantly in measures related to sensory features and autistic traits. Specifically, the P‐ASD group scored higher than the P‐CON group on the visual scores, tactile scores, activity level scores, auditory scores, and total scores of the AASP. They also had higher scores on the attention‐to‐detail subscale and total score of the AQ (Table 1 and Fig. 1).
Subsequently, we used the intraclass correlation coefficient (ICC) to quantify the consistency of these assessments within each child–parent pair. The ICC results showed significant consistency between ASD and their parents on the tactile scores, activity level scores, auditory scores, and total scores of the AASP, as well as in the total scores of the SORI (Table 2).
Table 2.
Intraclass correlation coefficients (ICC) for parent–child dyads for ASD and CON groups with corresponding 95% confidence intervals (CI)
| ASD (n = 27) & P‐ASD (n = 27) | CON (n = 21) & P‐CON (n = 21) | |||||||
|---|---|---|---|---|---|---|---|---|
| Variables | ICC | 95% CI | P | P permu | ICC | 95% CI | P | P permu |
| The AASP | ||||||||
| Taste/Smell | 0.44 | −0.24 to 0.74 | 0.08 | 0.06 | 0.53 | −0.11 to 0.81 | 0.04* | 0.08 |
| Movement | 0.43 | −0.26 to 0.74 | 0.08 | 0.09 | 0.54 | −0.53 to 0.81 | 0.04* | 0.04 |
| Visual | 0.33 | −0.47 to 0.69 | 0.16 | 0.14 | −0.09 | −1.73 to 0.56 | 0.57 | 0.42 |
| Tactile | 0.61 | 0.14 to 0.82 | 0.01* | <0.01* | 0.34 | −0.53 to 0.73 | 0.17 | 0.18 |
| Activity level | 0.61 | 0.15 to 0.82 | <0.01* | <0.01* | 0.02 | −0.81 to 0.54 | 0.48 | 0.45 |
| Auditory | 0.50 | −0.11 to 0.77 | 0.04* | 0.04* | −1.42 | −6.09 to 0.08 | 0.96 | 0.03 |
| Total scores | 0.72 | 0.39 to 0.87 | <0.01* | <0.01* | −0.15 | −1.57 to 0.52 | 0.63 | 0.37 |
| The SORI | 0.51 | −0.08 to 0.78 | 0.04* | 0.03* | −0.58 | −3.31 to 0.38 | 0.83 | 0.18 |
| The AQ | ||||||||
| Social skills | 0.64 | 0.16 to 0.84 | <0.01* | <0.01* | 0.30 | −0.79 to 0.72 | 0.22 | 0.22 |
| Attentional switch | −0.31 | −2.03 to 0.43 | 0.74 | 0.57 | −0.05 | −1.03 to 0.52 | 0.55 | 0.47 |
| Attention to detail | −0.11 | −1.57 to 0.52 | 0.60 | 0.46 | 0.14 | −1.26 to 0.66 | 0.37 | 0.38 |
| Communication | 0.54 | −0.06 to 0.80 | 0.03* | 0.03* | −0.07 | −1.78 to 0.57 | 0.56 | 0.47 |
| Imagination | 0.38 | −0.45 to 0.73 | 0.13 | 0.12 | −0.35 | −2.70 to 0.47 | 0.74 | 0.26 |
| Total scores | 0.40 | −0.39 to 0.74 | 0.12 | 0.08 | 0.01 | −1.45 to 0.60 | 0.49 | 0.49 |
Abbreviations: AASP, Adolescent/Adult Sensory Profile; AQ, Autism Spectrum Quotient; ASD, autism spectrum condition; CON, matched controls; P‐ASD, parents of children with ASD; P‐CON, parents of children with the CON; permu, permutation test; SORI, Sensory Over‐Responsivity Inventory.
P‐value <0.05.
We further validate the statistical significance of the observed ICC in both the ASD and CON families with a permutation approach (5000 times). The results revealed that only the ASD family showed significant ICC on the sensory profiles measured from questionnaires, suggesting that aberrant behavioral responses to sensory stimuli (hyper‐responsivity) are a heritable trait within ASD families.
Similar to their children with ASD, participants in the P‐ASD group exhibited a positive correlation between the AASP total scores and TAS‐20 scores (r = 0.41, P = 0.03) as well as a positive correlation between the total scores of the SORI and the RBS‐R (r = 0.46, P = 0.02), while there was no significant correlation found in the P‐CON group (all P >0.05).
Lab‐based sensory evaluations
ASD vs. CON. In the visual task, the ASD group reported more unpleasant feelings toward an 8 Hz flickering image than the CON group. In the auditory task, the ASD group, relative to the CON group, reported more feelings of unpleasantness to a 2000 Hz pure tone. In the tactile task, the ASD group, relative to the CON group, also reported more substantial unpleasantness ratings for both a plastic mesh material and a burlap fabric material (Fig. S3).
P‐ASD vs. P‐CON. In both the visual and the auditory tasks, participants in the P‐ASD group reported more substantial unpleasantness ratings than those in the P‐CON group. Individuals in the P‐ASD group reported more unpleasantness to a plastic mesh material and a burlap fabric material in the tactile task than those in the P‐CON group (Fig. S3).
Neurobiological aspects of the sensory profile
fMRI results
ASD vs. CON. Whole‐brain analyses showed that the ASD group had greater activation in response to visual stimuli (relative to null events) in the right superior frontal gyrus (SFG) and right lingual gyrus than the CON group. In contrast, the CON group, relative to the ASD group, showed greater activation in the left angular gyrus. In the auditory condition, the ASD group, compared to the CON group, had greater activation in the left superior temporal gyrus (STG), whereas the CON group showed greater activation in the right SFG, left middle cingulate cortex, and left insula. In the tactile condition, the ASD group had greater bilateral activation in the SI/SII, right medial frontal gyrus, and right inferior frontal gyrus than the CON group. In contrast, the CON group exhibited greater activation bilaterally in the supramarginal gyrus (Table S1 and Fig. 2a).
Fig. 2.
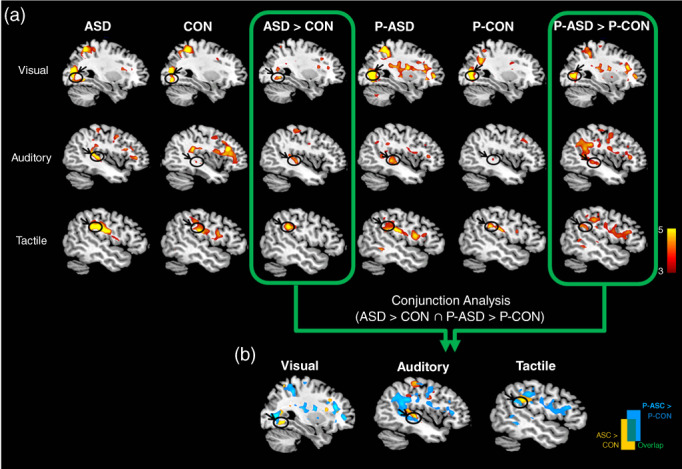
fMRI results. (a) Hemodynamic responses to sensory stimuli (visual, auditory, and tactile) within and between four groups. (b) Common areas of increased activations in two pairs of group‐difference maps for each (ASD > CON ∩ P‐ASD > P‐CON) are identified with conjunction analyses.
P‐ASD vs. P‐CON. In the visual condition, the P‐ASD group, relative to the P‐CON group, exhibited greater activation bilaterally in the anterior cingulate cortex, right lingual gyrus, and right insula. In contrast, the P‐CON group was associated with greater signal changes in the right angular gyrus. In the auditory condition, the P‐ASD group had greater activation in the right thalamus, the left SFG, the left STG, and the left insula than in the P‐CON group. In the tactile condition, the P‐ASD group had greater activation bilaterally in the SFG, bilaterally in the SI/SII, the right thalamus, and the left insula than the P‐CON group (Table S2 and Fig. 2a).
To identify the presence of an endophenotype at the neural level, we performed conjunction analyses separately for each sensory task. In the visual condition, the conjunction of group‐difference maps showed that greater activation was evident at the lingual gyrus when comparing the contrast between ASD and P‐ASD groups with the contrast between CON and P‐CON groups (ASD > CON ∩ P‐ASD > P‐CON). In the auditory and tactile conditions, overlapping activations were obtained in the STG and the SI/SII (Fig. 2b), respectively. These results indicate that individuals with ASD and their biological parents share similar hyperreactivity in sensory processing areas, which represents a neural endophenotype for ASD.
Correlations between the questionnaire‐based, the lab‐based, and the neural‐based sensory measurements
We then added the scores of sensory responsiveness into the regression model as a predictor of BOLD responses during each sensory condition. Specifically, the visual, auditory, and tactile scores of the AASP were used as the regressors of interest. In both the ASD and the P‐ASD groups, the visual, auditory, and tactile scores of the AASP positively correlated with activations in the primary visual cortex (visual condition), the STG (auditory condition), and the SI/SII (tactile condition), respectively. Moreover, higher unpleasantness ratings to visual (average of 1 and 8 Hz), auditory (average of 250 and 2000 Hz), and tactile (average for a plastic mesh material and a soft cosmetic brush) stimuli also positively correlated with activations in these sensory cortices in both ASD and P‐ASD groups.
The neural‐behavioral sensory profile of unaffected biological parents predicts children's AQ scores in high‐risk families for ASD
To further investigate the nature of the observed sensory profiles in the ASD families, we used a machine learning algorithm based on feedforward Back‐Propagation Artificial Neural Networks (BP‐ANN) to test the predictive power of the sensory profiles in the unaffected biological parents on the AQ scores in their offspring. In particular, we used four models to exploratively test the robustness of sensory signatures for AQ score prediction across different study cohorts. First, the prediction model from unaffected parent data (P‐ASD & P‐CON) to unseen data collected from children (ASD & CON) was validated by a leave‐children‐out approach that was performed by iteratively using parents' data for model training and the children's data for model testing (the parent‐to‐children model). Second, by the same token, the prediction model from children (ASD & CON) to unseen parent data (P‐ASD & P‐CON) was validated by using children's data for model training (ASD & CON), and the parent's data for the model testing (P‐ASD & P‐CON) (i.e., the children‐to‐parent model). Third, the generalizability of the prediction model was assessed by using high‐risk family data to train the model (P‐ASD & ASD) and the low‐risk family data for testing the model (P‐CON& CON) (i.e., the high risk‐to‐low risk model). Fourth, the generalizability of the low risk‐to‐high risk model was assessed by using low‐risk family data to train the model (P‐CON & CON) and the high‐risk family data for testing the model (P‐ASD & ASD). The parent‐to‐children model showed superior generalizability in predicting AQ scores among all four models. A significant correlation between actual AQ scores and the model‐predicted AQ scores was only found with the parent‐to‐children model (r = 0.47, P = 0.001, Fig. 3, Figs. S4 and S5, Table S3).
Fig. 3.
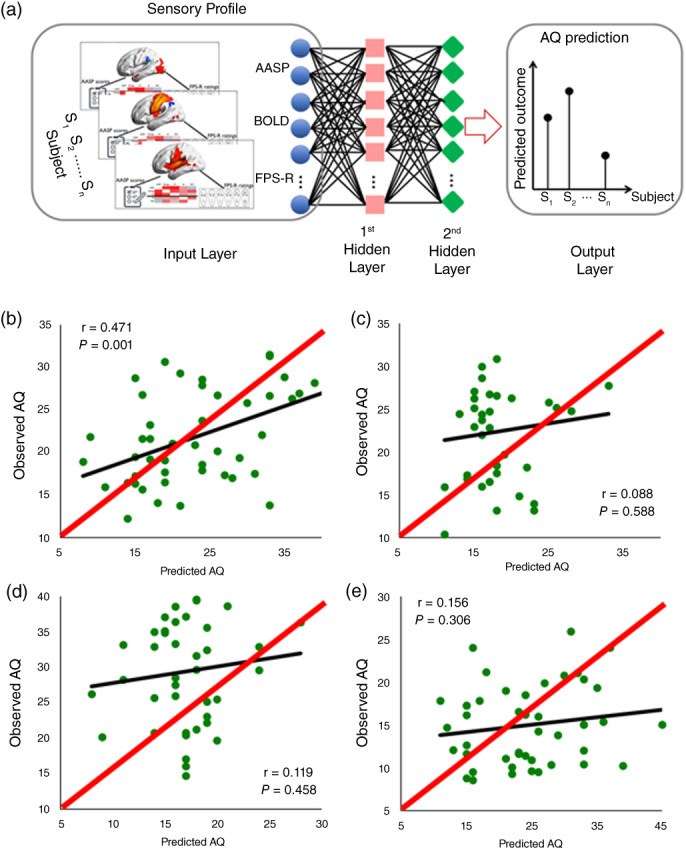
Prediction of children's AQ scores specific to the sensory profile of the unaffected biological parent using BP‐ANN. (a) The general structure of a fully connected feedforward back‐propagation artificial neural network (BP‐ANN). (b) Cross‐validation prediction of AQ scores using BP‐ANN on unaffected biological parent data to unseen data collected from children (r = 0.47, P = 0.001). The children‐to‐parent model (c), high‐risk‐to‐low‐risk model (d), and low‐risk‐to‐high‐risk model (e) failed to predict the outcome, demonstrating the specificity of the model for ASD prediction using a sensory profile from the unaffected biological parents. The red line represents a reference in a 2‐dimensional scatter plot comparing two data sets that should be identical under ideal conditions.
The neural‐behavioral sensory profile of unaffected biological parents predicts ASD diagnosis in their offspring
A multivariate pattern classification using the LibSVM Classifier was also tested to investigate how well the sensory profile of the unaffected biological parent predicted ASD diagnosis in their offspring. Similar to the robustness test of BP‐ANN models, performances of the four predictive models (i.e., the parent‐to‐children model, children‐to‐parent model, high risk‐to‐low risk model, and low risk‐to‐high risk model) were assessed by using unseen data collected from children, parents, low‐risk family, and high‐risk family. Furthermore, to test whether the predictive validity of the proposed parent‐to‐children model was significantly superior against the chance level of 50%, we created a baseline parent dataset in which the parents' labels (high‐risk/P‐ASD vs. low‐risk/P‐CON) were randomly shuffled. The prediction accuracy (ACC) of the parent‐to‐children model, children‐to‐parent model, high‐to‐low risk model, low‐to‐high risk model, and the baseline model was 71.4%, 70.24%, 49.48%, 49.37%, and 51.18%, respectively. However, the parent‐to‐children model and the children‐to‐parent model had better prediction ACC with a P‐value less than 0.0001 against the high risk‐to‐low risk model, low risk‐to‐high risk model, and the chance level of 50% (P Bonferroni corrected <0.05), these two models are indistinguishable in terms of the diagnostic predictability, indicating highly heritable features in the sensory profile for ASD (Fig. S6).
Discussion
In the present study, we identify that alterations in sensory responsivity are significant features representing the neural endophenotypes of ASD. At the behavioral level, the ASD and the P‐ASD groups showed hyper‐responsivity to sensory stimuli compared to the CON and the P‐CON groups. Consistency of the sensory profiles of behaviors was only observed within the ASD parent–child dyads. At the neural level, the ASD group, relative to the CON group, showed stronger activation within the sensory cortices in response to the sensory stimuli. Our fMRI results further indicate that the response within these sensory cortices differs significantly between the P‐ASD and the P‐CON individuals. Altogether, these findings reveal that genetic susceptibilities for ASD may contribute to behavioral and neural patterns of sensory hyper‐responsivity. In addition, we developed machine learning models using neural‐behavioral sensory profiles from four independent study cohorts (ASD, CON, P‐ASD, and P‐CON, n = 104) and obtained robust prediction performances of AQ scores and ASD diagnoses.
To our knowledge, no prior research has used multimethod approaches (i.e., questionnaire‐based, lab‐based, and neural‐based sensory measures) to examine whether sensory differences can be endophenotypes for ASD. Hyper‐responsivity to sensory stimuli is one of the significant characteristics of ASD and is well documented in the scientific literature. 37 , 38 Here, our results support the finding that individuals with ASD report sensory hyper‐responsivity overall and in most sensory modalities and experience strong unpleasantness toward sensory stimuli. Moreover, our behavioral data also aligns with previous findings stating that parents of children with ASD display greater sensory hyper‐responsivity than the general population. 19 , 22 , 23 , 39 In line with the data from a recent study, 22 we observed moderate to high levels of agreement on several sensory domain scores within ASD parent–child dyads. More importantly, we performed nonparametric permutation tests to examine whether our observed statistics (within‐pair correlations) occurred by chance. We found that significant agreements were obtained on tactile subscale scores, activity level subscale scores, auditory subscale scores, and the total scores of the AASP, as well as the SORI total scores within ASD families only. These data strengthen the rationale for the search for endophenotypes in ASD and reveal that altered sensory responsiveness may be heritable solely within ASD families.
Compared to matched controls, the current study is the first work to investigate the neural patterns of sensory responsivity in dyads of individuals with ASD and their unaffected biological parents. For individuals with ASD, our results were consistent with recent fMRI‐based research 34 , 35 , 40 in that the ASD group relative to the CON group had increased BOLD responses to multiple sensory stimuli (visual, auditory, and tactile) within the sensory cortices (i.e., the lingual gyrus, the STG, and the SI/SII), irrespective of the sensory load. In addition, the level of activity in these sensory regions was positively correlated with both the sensory domain scores and unpleasantness ratings to sensory stimuli, suggesting that group differences (ASD vs. CON) are related to higher levels of sensory hyper‐responsivity in ASD.
Notably, the parent‐to‐children predictive model, built upon the sensory signature of unaffected biological parents to predict the AQ score and ASD diagnosis on unseen data collected from children, exhibited a superior generalizability in the prediction of ASD in their offspring, as compared to other control models trained on low familial risk for ASD data. Machine learning approaches corroborated the findings of highly heritable features within the sensory profile of ASD, evinced by the parametric analyses and literature findings. These results offer optimistic possibilities to further bring forward an ASD diagnosis even before the birth of the offspring by using the sensory signature of the biological parents, especially for families with a high risk of ASD.
There are some limitations worth noting for the current study. First, the reported prediction model for ASD diagnosis remains to be replicated in an entirely independent sample. More so, it is not known whether this sensory signature predicts outcomes within a broader class among the autism spectrum. Second, the sample size for each of our four independent study cohorts is not to be considered a big dataset. As a similar challenge faced with many other studies, using a smaller sample size in training machine learning models brings the potential for overfitting. This situation arises when a model is excessively tailored to the training set, leading to subpar performance on unfamiliar data. In our study, we attempted to mitigate this through cross‐validation on data from varied cohorts, which improved the robustness of our predictive models. However, to truly validate the applicability of the sensory signature in ASD, subsequent studies involving larger samples are essential. In addition to concerns about a small sample size, the imbalanced gender distribution in the offspring and parent groups may also influence the predictability of the sensory signature. The greater number of males diagnosed with ASD is consistent with the majority of autism research literature. Conversely, the higher number of females in parent groups can be attributed to the observation that mothers typically are the primary caregivers and their higher motivation to participate in autism‐related research and support groups. While we controlled for gender in both parent and offspring groups, future studies focusing on the cross‐generational gender effects in ASD prediction are essential. Thirdly, our findings might be limited in terms of result generalization due to our selection criteria: (1) we only included ASD participants with an IQ above 80 to ensure their ability to cope with the fMRI setting and to meet the demands of the entire experiment, and (2) this study did not use confirmatory evaluation tools for ASD like the Social Communication Questionnaire, 41 Autism Diagnostic Interview‐Revised, 42 or Autism Diagnostic Observation Schedule. 43 This might raise concerns that the ASD individuals may fall in the less severe end of the spectrum, which could potentially lead to a conservative estimation of the actual effects. Nevertheless, the DSM‐5 ASD severity classification system has been demonstrated to be correlated with the calibrated severity scores from the ADOS–Second Edition. 44 In addition, considering that more pronounced sensory processing abnormalities were reportedly to be associated with more severe autism symptoms, 17 , 34 , 35 , 45 , 46 , 47 , 48 , 49 the predictability effect of sensory signatures might be larger for individuals with more severe autism symptoms.
In summary, through the present research, we developed behavioral‐and‐fMRI‐optimized deep learning and classification models capable of robustly predicting ASD outcomes by using sensory abnormalities. Furthermore, these models were also able to distinguish between low familial risk for ASD and high familial risk for ASD at the individual subject level, which may further support early identification and diagnosis of ASD. Together, these findings break down individual‐level multidimensional sensory profiles, where a genotype–phenotype association is obscured within the broader clinical diagnosis of ASD and its associated biological heterogeneity and lays a path toward machine‐learning‐driven personalized approaches for early detection of ASD.
Author contributions
Y‐T.F., C.C., and Y.C. conceived and conceptualized the study. Y‐T.F., C.C., C‐T.W., C‐H.C., and C‐C.W. collected and analyzed the data. Y‐T.F., C.C., and Y.C. conducted the necessary literature reviews and drafted the first manuscript. C‐T.W., C‐M.H., R.M., and O.J.L.T. provided critical feedback and helped shape the manuscript. All authors contributed toward the revision and writing the final draft.
Disclosure statement
The authors declared no potential conflicts of interest concerning this article's research, authorship, and/or publication.
Supporting information
Data S1. 1. Supplementary Materials and Methods.
1.1 Questionnaire‐based evaluations
1.2 Lab‐based sensory evaluations
1.3. Functional MRI (fMRI) experiment
1.3.1 fMRI sensory task paradigm
1.3.2. fMRI data acquisition, image processing, and analysis
2. Results
2.1 Results from the questionnaire‐based assessments
2.2 Results from the lab‐based sensory evaluations
2.3 ROIs results
3. Machine Learning Analysis
3.1 Artificial Neural Network (ANN) structure
3.2 LibSVM Classifier
Table S1. Group differences (ASD vs CON) in neural activation during the sensory tasks.
Table S2. Group differences (P‐ASD vs P‐CON) in neural activation during the sensory tasks.
Table S3. Minimizing the mean square (MSE) error results and model selection.
Fig. S1. Illustration of the lab‐based sensory evaluations.
Fig. S2. Illustration of the stimuli used in the fMRI experiment.
Fig. S3. Comparisons of unpleasantness ratings to sensory stimuli between children groups and between parents groups.
Fig. S4. MSE curves for training, evaluation, and testing in the prediction model of unaffected parent data.
Fig. S5. The AQ score prediction showed superior generalizability of the parent‐to‐children model.
Fig. S6. The predicted ACC for the diagnostic label is from the parent‐to‐children model, children‐to‐parent model, high‐to‐low‐risk model, low‐to‐high‐risk model, and the baseline model.
Acknowledgments
This work was financially supported by the National Science and Technology Council (106‐2410‐H‐009‐059‐MY2; 108‐2410‐H‐009‐020‐MY3; 112‐2410‐H‐038‐029; 112‐2636‐H‐038‐005) and the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan (DP2‐TMU‐112‐N‐08).
References
- 1. Centers for Disease Control and Prevention . Data and statistics on children's mental health. 2022. Available from: www.cdc.gov/childrensmentalhealth/index.html.
- 2. Hazlett HC, Gu H, Munsell BC et al. Early brain development in infants at high risk for autism spectrum disorder. Nature 2017; 542: 348–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Goldsmith HH, Van Hulle CA, Arneson CL, Schreiber JE, Gernsbacher MA. A population‐based twin study of parentally reported tactile and auditory defensiveness in young children. J. Abnorm. Child Psychol. 2006; 34: 393–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. DeLorey TM, Sahbaie P, Hashemi E, Li WW, Salehi A, Clark DJ. Somatosensory and sensorimotor consequences associated with the heterozygous disruption of the autism candidate gene, Gabrb3. Behav. Brain Res. 2011; 216: 36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Penagarikano O, Abrahams BS, Herman EI et al. Absence of CNTNAP2 leads to epilepsy, neuronal migration abnormalities, and core autism‐related deficits. Cell 2011; 147: 235–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tavassoli T, Auyeung B, Murphy LC, Baron‐Cohen S, Chakrabarti B. Variation in the autism candidate gene GABRB3 modulates tactile sensitivity in typically developing children. Mol. Autism. 2012; 3: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Holt RJ, Chura LR, Lai MC et al. 'Reading the mind in the Eyes': An fMRI study of adolescents with autism and their siblings. Psychol. Med. 2014; 44: 3215–3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sandin S, Lichtenstein P, Kuja‐Halkola R, Hultman C, Larsson H, Reichenberg A. The heritability of autism Spectrum disorder. Jama 2017; 318: 1182–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tick B, Bolton P, Happe F, Rutter M, Rijsdijk F. Heritability of autism spectrum disorders: A meta‐analysis of twin studies. J. Child Psychol. Psychiatry 2016; 57: 585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bailey A, Palferman S, Heavey L, Le Couteur A. Autism: The phenotype in relatives. J. Autism Dev. Disord. 1998; 28: 369–392. [DOI] [PubMed] [Google Scholar]
- 11. Constantino JN, Lajonchere C, Lutz M et al. Autistic social impairment in the siblings of children with pervasive developmental disorders. Am. J. Psychiatry 2006; 163: 294–296. [DOI] [PubMed] [Google Scholar]
- 12. Gerdts J, Bernier R. The broader autism phenotype and its implications on the etiology and treatment of autism spectrum disorders. Autism Res. Treat. 2011; 2011: 545901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rutter M. Genetic studies of autism: From the 1970s into the millennium. J. Abnorm. Child Psychol. 2000; 28: 3–14. [DOI] [PubMed] [Google Scholar]
- 14. Sucksmith E, Roth I, Hoekstra RA. Autistic traits below the clinical threshold: re‐examining the broader autism phenotype in the 21st century. Neuropsychol. Rev. 2011; 21: 360–389. [DOI] [PubMed] [Google Scholar]
- 15. Kanner L. Autistic disturbances of affective contact. Nervous Child. 1943; 2: 217–250. [PubMed] [Google Scholar]
- 16. Asperger H. Die“Autistischen psychopathen”im kindesalter. Arch. Psychiatr. Nervenkr. 1944; 117: 76–136. [Google Scholar]
- 17. Marco EJ, Hinkley LB, Hill SS, Nagarajan SS. Sensory processing in autism: a review of neurophysiologic findings. Pediatr. Res. 2011; 69: 48R–54R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders, 5th edn. American Psychiatric Association, Washington, DC, 2013. [Google Scholar]
- 19. Donaldson CK, Stauder JEA, Donkers FCL. Increased sensory processing Atypicalities in parents of multiplex ASD families versus typically developing and simplex ASD families. J. Autism Dev. Disord. 2017; 47: 535–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. De la Marche W, Steyaert J, Noens I. Atypical sensory processing in adolescents with an autism spectrum disorder and their non‐affected siblings. Res. Autism Spectr. Disord. 2012; 6: 639–645. [Google Scholar]
- 21. Germani T, Zwaigenbaum L, Bryson S et al. Brief report: Assessment of early sensory processing in infants at high‐risk of autism spectrum disorder. J. Autism Dev. Disord. 2014; 44: 3264–3270. [DOI] [PubMed] [Google Scholar]
- 22. Glod M, Riby DM, Honey E, Rodgers J. Sensory atypicalities in dyads of children with autism spectrum disorder (ASD) and their parents. Autism Research: Official Journal of the International Society for Autism Research. 2017; 10: 531–538. [DOI] [PubMed] [Google Scholar]
- 23. Uljarevic M, Prior MR, Leekam SR. First evidence of sensory atypicality in mothers of children with autism Spectrum disorder (ASD). Mol. Autism. 2014; 5: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wechsler D. Manual for the Wechsler Abbreviated Scale of Intelligence. Psychological Corporation, San Antonio, TX, 1999. [Google Scholar]
- 25. Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 1971; 9: 97–113. [DOI] [PubMed] [Google Scholar]
- 26. Baron‐Cohen S, Hoekstra RA, Knickmeyer R, Wheelwright S. The autism‐Spectrum quotient (AQ)‐adolescent version. J. Autism Dev. Disord. 2006; 36: 343–350. [DOI] [PubMed] [Google Scholar]
- 27. Baron‐Cohen S, Wheelwright S, Skinner R, Martin J, Clubley E. The autism‐spectrum quotient (AQ): Evidence from asperger syndrome/high‐functioning autism, males and females, scientists and mathematicians. J. Autism Dev. Disord. 2001; 31: 5–17. [DOI] [PubMed] [Google Scholar]
- 28. Brown C, Dunn W. Adolescent/Adult Sensory Profile. Psychological Corporation, San Antonio, TX, 2002; 2002. [Google Scholar]
- 29. Tseng MH, Chen WS. Chinese Version of Adolescent‐Adult Sensory Profile: User's Manual. Chinese Behavioral Science Corporation, Taipei, 2009. [Google Scholar]
- 30. Schoen SA, Miller LJ, Green KE. Pilot study of the sensory over‐responsivity scales: Assessment and inventory. Am. J. Occup. Ther. 2008; 62: 393–406. [DOI] [PubMed] [Google Scholar]
- 31. Bodfish JW, Symons FJ, Parker DE, Lewis MH. Varieties of repetitive behavior in autism: Comparisons to mental retardation. J. Autism Dev. Disord. 2000; 30: 237–243. [DOI] [PubMed] [Google Scholar]
- 32. Bagby RM, Parker JD, Taylor GJ. The twenty‐item Toronto alexithymia scale‐I. Item selection and cross‐validation of the factor structure. J. Psychosom. Res. 1994; 38: 23–32. [DOI] [PubMed] [Google Scholar]
- 33. Bagby RM, Taylor GJ, Parker JD. The twenty‐item Toronto alexithymia scale‐II. Convergent, discriminant, and concurrent validity. J. Psychosom. Res. 1994; 38: 33–40. [DOI] [PubMed] [Google Scholar]
- 34. Green SA, Rudie JD, Colich NL et al. Overreactive brain responses to sensory stimuli in youth with autism spectrum disorders. J. Am. Acad. Child Adolesc. Psychiatry 2013; 52: 1158–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Green SA, Hernandez L, Tottenham N, Krasileva K, Bookheimer SY, Dapretto M. Neurobiology of sensory Overresponsivity in youth with autism Spectrum disorders. JAMA Psychiatry 2015; 72: 778–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chang CC, Lin CJ. LIBSVM: A library for support vector machines. ACM Transactions on Intelligent Systems and Technology (TIST). 2011; 2: 1–27. [Google Scholar]
- 37. Baum SH, Stevenson RA, Wallace MT. Behavioral, perceptual, and neural alterations in sensory and multisensory function in autism spectrum disorder. Prog. Neurobiol. 2015; 134: 140–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Robertson CE, Baron‐Cohen S. Sensory perception in autism. Nat. Rev. Neurosci. 2017; 18: 671–684. [DOI] [PubMed] [Google Scholar]
- 39. Uljarevic M, Carrington S, Leekam S. Brief report: Effects of sensory sensitivity and intolerance of uncertainty on anxiety in mothers of children with autism Spectrum disorder. J. Autism Dev. Disord. 2016; 46: 315–319. [DOI] [PubMed] [Google Scholar]
- 40. Cascio CJ, Moana‐Filho EJ, Guest S et al. Perceptual and neural response to affective tactile texture stimulation in adults with autism spectrum disorders. Autism Res. 2012; 5: 231–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rutter M, Bailey A, Lord C. The Social Communication Questionnaire: Manual. Western Psychological Services, Los Angeles, 2003. [Google Scholar]
- 42. Lord C, Rutter M, Le Couteur A. Autism diagnostic interview‐revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J. Autism Dev. Disord. 1994; 24: 659–685. [DOI] [PubMed] [Google Scholar]
- 43. Kim S, Lord C. Autism diagnostic observation schedule. In: Kreutzer J, DeLuca J, Caplan B (eds). Encyclopedia of Clinical Neuropsychology. Springer, New York, 2011. [Google Scholar]
- 44. Mazurek MO, Lu F, Macklin EA, Handen BL. Factors associated with DSM‐5 severity level ratings for autism spectrum disorder. Autism 2019; 23: 468–476. [DOI] [PubMed] [Google Scholar]
- 45. Tavassoli T, Miller LJ, Schoen SA, Nielsen DM, Baron‐Cohen S. Sensory over‐responsivity in adults with autism spectrum conditions. Autism 2014; 18: 428–432. [DOI] [PubMed] [Google Scholar]
- 46. Baron‐Cohen S, Ashwin E, Ashwin C, Tavassoli T, Chakrabarti B. Talent in autism: Hyper‐systemizing, hyper‐attention to detail and sensory hypersensitivity. Philosophical Transactions of the Royal Society B: Biological Sciences. 2009; 364: 1377–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hilton CL, Harper JD, Kueker RH et al. Sensory responsiveness as a predictor of social severity in children with high functioning autism spectrum disorders. J. Autism Dev. Disord. 2010; 40: 937–945. [DOI] [PubMed] [Google Scholar]
- 48. Tavassoli T, Hoekstra RA, Baron‐Cohen S. The sensory perception quotient (SPQ): Development and validation of a new sensory questionnaire for adults with and without autism. Mol. Autism. 2014; 5: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tavassoli T, Miller LJ, Schoen SA, Brout JJ, Sullivan J, Baron‐Cohen S. Sensory reactivity, empathizing and systemizing in autism spectrum conditions and sensory processing disorder. Dev. Cogn. Neurosci. 2018; 29: 72–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. 1. Supplementary Materials and Methods.
1.1 Questionnaire‐based evaluations
1.2 Lab‐based sensory evaluations
1.3. Functional MRI (fMRI) experiment
1.3.1 fMRI sensory task paradigm
1.3.2. fMRI data acquisition, image processing, and analysis
2. Results
2.1 Results from the questionnaire‐based assessments
2.2 Results from the lab‐based sensory evaluations
2.3 ROIs results
3. Machine Learning Analysis
3.1 Artificial Neural Network (ANN) structure
3.2 LibSVM Classifier
Table S1. Group differences (ASD vs CON) in neural activation during the sensory tasks.
Table S2. Group differences (P‐ASD vs P‐CON) in neural activation during the sensory tasks.
Table S3. Minimizing the mean square (MSE) error results and model selection.
Fig. S1. Illustration of the lab‐based sensory evaluations.
Fig. S2. Illustration of the stimuli used in the fMRI experiment.
Fig. S3. Comparisons of unpleasantness ratings to sensory stimuli between children groups and between parents groups.
Fig. S4. MSE curves for training, evaluation, and testing in the prediction model of unaffected parent data.
Fig. S5. The AQ score prediction showed superior generalizability of the parent‐to‐children model.
Fig. S6. The predicted ACC for the diagnostic label is from the parent‐to‐children model, children‐to‐parent model, high‐to‐low‐risk model, low‐to‐high‐risk model, and the baseline model.


