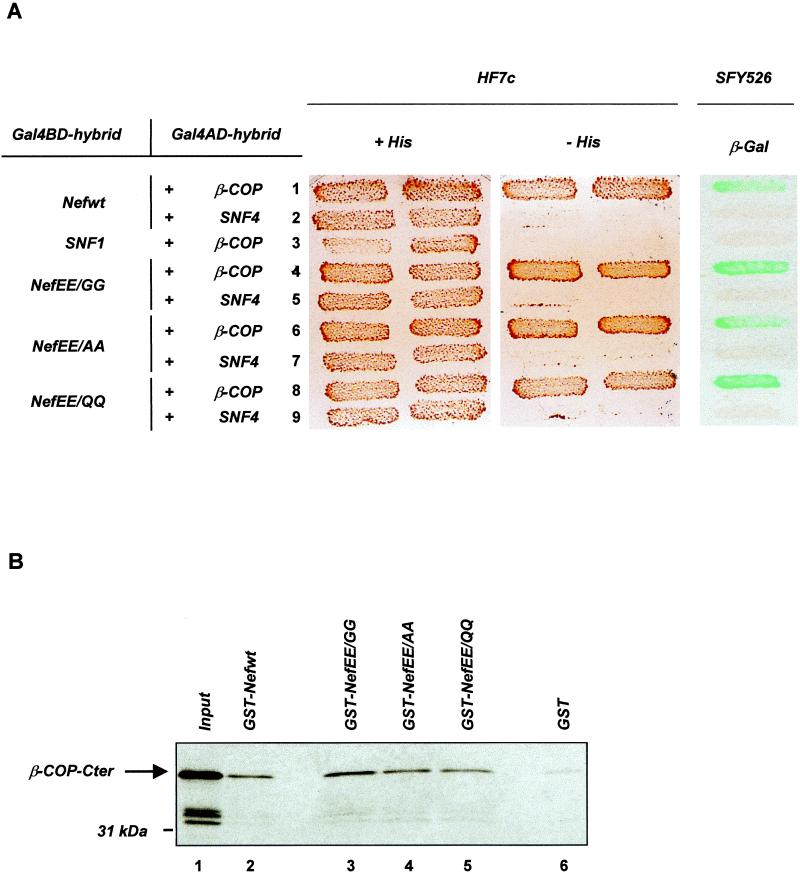
FIG. 1.
Mutation of the di-Glu sequence does not affect the binding of Nef to β-COP. (A) Nef binding to β-COP in a two-hybrid assay. HF7c and SFY526 yeast strains expressing the indicated pairs of Gal4BD and Gal4AD hybrid proteins were analyzed for histidine auxotrophy and β-Gal activity, respectively. HF7c double transformants were patched on selective medium with histidine (left) and then replica plated on histidine-free medium (middle). SFY526 double transformants were patched on selective medium and then replica plated on a Whatman filter for detection of β-Gal activity (right). Growth on histidine-free medium and expression of β-Gal indicate interaction between hybrid proteins. Binding specificity was verified by the absence of interaction between β-COP and SNF1 (lane 3) and between wt or mutated Nef and SNF4 (lanes 2, 5, 7, and 9). (B) In vitro binding of Nef to β-COP. [35S]-β-COP-Cter synthesized in vitro was incubated with equal amounts of GST (lane 6) or GST-Nef fusion proteins (lanes 2 to 5) immobilized on glutathione-agarose beads. Bound labeled material was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and autoradiography. One-fifth of the [35S]-β-COP-Cter input was run on lane 1.