Abstract
Background
Intracranial bleeding (ICB) is a serious complication during veno-venous extracorporeal membrane oxygenation (V-V ECMO), with potentially fatal consequences.
Purpose
This study aimed to evaluate the incidence, time of detection of ICB among patients treated with V-V ECMO and potential risk factors for developing ICB during V-V ECMO.
Methods
Five hundred fifty six patients were included in this retrospective single center analysis.
Results
Median time on V-V ECMO was 9 (IQR 6-15) days. Intracranial bleeding during V-V ECMO was detected in 10.9% of all patients (61 patients with ICB). Only 17 patients with ICB presented obvious clinical symptoms. Intracranial bleeding was detected on cerebral imaging in median after 5 days (IQR 1-14) after starting V-V ECMO. Overall survival to hospital discharge was 63.7% (ICB: 29.5%). Risk factors of ICB before starting V-V ECMO in univariable analysis were platelets <100/nl (OR: 3.82), creatinine >1.5mg/dl (OR: 1.98), norepinephrine >2.5mg/h (OR: 2.5), ASAT >80U/L (OR: 1.86), blood-urea >100mg/dl (OR: 1.81) and LDH >550u/L (OR: 2.07). Factors associated with cannulation were rapid decrease in paCO2 >35mmHg (OR: 2.56) and rapid decrease in norepinephrine >1mg/h (OR: 2.53). Multivariable analysis revealed low platelets, high paCO2 before ECMO, and rapid drop in paCO2 after V-V ECMO initiation as significant risk factors for ICB.
Conclusion
The results emphasize that ICB is a frequent complication during V-V ECMO. Many bleedings were incidental findings, therefore screening for ICB is advisable. The univariate risk factors reflect the underlying disease severity, coagulation disorders and peri-cannulation factors, and may help to identify patients at risk.
Keywords: veno-venous extracorporeal membrane oxygenation, intracranial bleeding, intracranial hemorrhage, incidence, risk factors
Background
Veno-venous extracorporeal membrane oxygenation (V-V ECMO) is an increasingly used option of organ support in cases of severe respiratory failure.1–4
Thromboembolic complications are numerous during organ support with ECMO,5,6 thus anticoagulation therapy is necessary. However, bleeding complications during ECMO support are frequent as well, 6 and the most critical bleeding complication during V-V ECMO is intracranial bleeding (ICB).6–8 Intracranial bleeding has severe implications on both survival and further quality of life.9–14 According to the Extracorporeal Life Support Organization (ELSO) and recent studies, ICB occurs in 2.5% up to 16.4%3,6,12,13,15,16 of patients. Intracranial bleeding is described as the most common type of neurological injury during V-V ECMO.6,12 Besides the influence of the ECMO circuit and the use of anticoagulants, other mechanisms might also increase the risk of ICB in these patients. The severity of underlying disease contributes to ICB during V-V ECMO support as well. 17
Due to the increased use of V-V ECMO and the absence of a common screening algorithm to identify ICB, the frequency of ICB might be underestimated in register trials since subclinical bleedings might be missed. Thus, the detection of ICB has important clinical consequences with respect to further management and anticoagulation.
The aim of this study is to investigate the frequency and timing of ICB during V-V ECMO, the impact on mortality and identification of potential risk factors for developing ICB among patients that underwent screening for ICB during organ support with V-V ECMO.
Methods
Patients
We used the ECMO database of the University Hospital Regensburg (UKR) for this retrospective analysis. The database records important clinical information on all ECMO patients (patient-related data, ECMO characteristics, ventilator settings, medication, hemodynamic data, laboratory values and complications). All consecutive patients supported with V-V ECMO at the UKR between December 2006 and March 2019 were eligible for this analysis.
All patients included met the following inclusion criteria: availability of at least one cerebral imaging (cerebral computed tomography (cCT) or cerebral magnetic resonance imaging (cMRI)) obtained during or after ECMO support. All cMRI scans were performed after V-V ECMO removal. Patients were excluded if there was evidence of cerebral bleeding prior to ECMO or ICB due to trauma. Traumatic ICB was considered if medical history reporting stated a history of trauma. In case of medical admission, trauma was not considered. All cerebral imagings and CT scans were clinically indicated.
Extracorporeal membrane oxygenation management and early cerebral CT procedures at our institution
Patient selection for V-V ECMO at our center is done according to the EOLIA criteria. 18 At cannulation, UFH dosing is determined on an individual basis based on clinical evidence of bleeding, ongoing anticoagulation therapy and laboratory testing. If active bleeding is ongoing, platelets are below 100,000/nl, therapeutic anticoagulation is already established or laboratory tests indicate severe coagulation disorder (e.g., aPTT longer than 45 s or fibrinogen below 150mg/dl), a bolus of UFH is not given. In the other cases, a bolus of up to 5,000IE UFH is given after successful vessel puncture.
During ECMO support, the standard of care at our institution is a continuous intravenous infusion of UFH or argatroban for systemic anticoagulation, aiming for a 1.5 to 1.8-fold increase (resp. 50 – 60s) in the activated partial thromboplastin time (aPTT). The aPTT and dose of UFH/argatroban are monitored and adjusted daily, or more frequently if necessary. When major bleeding occurs, anticoagulation is stopped and restarted once the bleeding is under control. In case of minor bleeding, local therapies are used, anticoagulation is stopped if bleeding persists despite local therapy, and the best treatment stragegy is then determined on an individual basis. In the case of non-fatal ICB, the size of the ICB is controlled after 24–48 h and anticoagulation is restarted based on individual determination. All patients undergo arterial blood gas analysis (ABG) before V-V ECMO cannulation (pre-ECMO values) and 2 h after initiation. Each morning during V-V ECMO support, ABG, ventilator settings, as well as hematological, biochemical, and coagulative blood markers are recorded. Veno-venous extracorporeal membrane oxygenation configuration, pumps, oxygenators and cannula diameter are presented in the Supplement material.
In our institution, a first CT scan is performed soon after V-V ECMO cannulation (on the first or second day). If a CT scan is performed immediately prior to V-V ECMO cannulation, or the patient is too unstable for transportation, the first CT scan with V-V ECMO is done within a week of V-V ECMO support.
This CT scan includes cerebral, chest, abdominal and thigh imaging to verify correct positions of the cannulas, to screen for focus of infection, unrecognized hemorrhage or malignancies, and to identify early signs of cerebral ischemia or hemorrhage that may not yet be clinically evident. Although reasonable, the approach of doing an early CT scan after cannulation is eminence-based at present. Further cCT scans are performed when performing CT (chest or abdominal) due to other pertinent reasons, or if patients exhibit new neurologic abnormalities or have persistent neurological disability.
Analysis of cerebral CT images
Formal radiologists’ description and reports of cerebral imaging were screened to identify evidence of cerebral pathology, namely: intraparenchymal hemorrhage, subarachnoid hemorrhage, subdural hemorrhage, or epidural hemorrhage. Each intracerebral bleeding was documented as left, right or bi-hemispheric.
Only the primary manifestation of a cerebral complication was categorized, while a secondary hemorrhagic transformation was classified as primary ischemic and not included in this analysis. Reports describing ICB were reviewed and reevaluated in collaboration with a radiologist.
Statistical analyses, laboratory tests and definitions
The primary aim of this study was to estimate the frequency of ICB during organ support with V-V ECMO without an underlying hypothesis. Thus, no a priori sample size calculation was performed. Instead, as many patients as possible were included in this retrospective trial.
Descriptive statistics are presented with number and percentage for categorical variables, and with median 25th – 75th percentile interquartile range (IQR) for continuous variables. Between-group comparisons of categorical and metric variables were performed using Fisher’s exact test, or Chi-square test of independence as indicated, or the Mann-Whitney U-test. Changes over time (paired samples) were analyzed using the Wilcoxon signed rank test. Univariate and multivariable logistic regression analyses were used to identify risk factors for ICB during V-V ECMO. Factors included in the calculation of risk factors were considered significantly different between the groups at baseline before starting V-V ECMO support at p < 0.05 (Table 1).
Table 1.
Patient characteristics before starting V-V ECMO. For paCO2 and norepinephrine, the change from pre-cannulation to 2 h after starting V-V ECMO (Delta-values) is presented. Data are shown as median and IQR (25th-75th).
| Variable | All N = 556 median (IQR 25th-75th) | ICB N = 61 median (IQR 25th-75th) | No ICB N = 495 median (IQR 25th-75th) | p-value |
|---|---|---|---|---|
| General data | ||||
| Age | 52.9 (41.3–62.1) | 55.4 (46.1–66.2) | 52.7 (40.7–61.8) | 0.071 |
| Male sex n (%) | 368 (66) | 41 (67) | 327 (66) | 0.85 |
| Survival to hospital discharge n (%) | 354 (64) | 18 (30) | 336 (68) | <0.001 |
| ECMO duration (days) | 9 (6–15) | 9 (6–16) | 9 (6–15) | 0.856 |
| SOFA score | 13 (10–16) | 16 (13–18) | 13 (10–16) | <0.001 |
| Antiplatelet therapy n (%) | 7 (1.3) | 1 (1.6) | 6 (1.2) | 0.778 |
| ECMO Indication | 0.155 | |||
| Bacterial pneumonia n (%) | 271 (49) | 32 (52) | 239 (48) | |
| Viral pneumonia n (%) | 75 (13) | 8 (13) | 67 (14) | |
| ALF after trauma n (%) | 34 (6) | 1 (2) | 33 (7) | |
| ALF after surgery n (%) | 96 (17) | 9 (15) | 87 (18) | |
| ALF after chemotherapy n (%) | 13 (2) | 4 (7) | 9 (2) | |
| Other n (%) | 67 (12) | 7 (11) | 60 (12) | |
| Laboratory Testing | ||||
| Impaired kidney function (creatinine >1.5mg/dl) before ECMO treatment n (%) | 218 (39) | 33 (54) | 185 (37) | 0.012 |
| aPTT (s) | 42 (35–55) | 46 (39.5–61.5) | 42 (35–55) | 0.024 |
| Fibrinogen (mg/dl) | 500 (342–644) | 515 (352–646) | 500 (339–644.5) | 0.753 |
| Platelets (/nl) | 173 (100–269) | 98 (47–212) | 182.5 (113–275) | <0.001 |
| Blood-urea (mg/dl) | 58 (38–98) | 65 (47–130) | 57 (36–95) | 0.006 |
| Bilirubin (mg/dl) | 0.8 (0.5–1.9) | 0.8 (0.6–2.4) | 0.8 (0.5–1.9) | 0.244 |
| ASAT (U/L) | 689 (36–152) | 103 (43–208) | 66 (34–14) | 0.036 |
| LDH (U/L) | 418 (274–676) | 550 (324–900) | 408 (270–651) | 0.002 |
| Ventilator Settings and Blood Gas Analysis | ||||
| PEEP (mbar) | 15 (12–17) | 15 (13–18) | 15 (12–17) | 0.475 |
| PaCO2 (mmHg) | 64 (51–78) | 71 (57–88) | 62 (50–77) | 0.009 |
| PaO2 (mmHg) | 67 (56–81) | 68 (58–77) | 66 (56–82) | 0.901 |
| P/F ratio (mmHg) | 70 (56–93) | 70 (60–84) | 69 (56–94) | 0.852 |
| pH | 7.22 (7.15–7.30) | 7.2 (7.11–7.28) | 7.23 (7.15–7.31) | 0.094 |
| pMax (mbar) | 35 (30–38) | 35 (30–38) | 34 (30–38) | 0.315 |
| Hemdoynamics before starting V-V ECMO | ||||
| Norepinephrine (mg/h) | 1.6 (0.6–3) | 2.5 (0.8–4.9) | 1.5 (0.6–3) | 0.001 |
| MAP (mmHg) | 68 (61–76) | 67 (61–79) | 68 (61–76) | 0.663 |
| Delta values (pre ECMO - 2 hours after starting V-V ECMO) | ||||
| Delta norepinephrine (pre- (2h)) (mg/h) | 0.4 (0–1.1) | 1 (0.1–2.3) | 0.4 (0–1) | 0.005 |
| Delta paCO2 (pre- (2h)) (mmHg) | 26 (15–41) | 37 (19–49) | 26 (14–40) | 0.007 |
SOFA: Sequential organ failure assessment; ALF: Acute Lung Failure; aPTT: activated partial Thromboplastin-Time; ASAT: Aspartat-Aminotransferasis; LDH: Lactate-Dehydrogenasis; PEEP: Positive End-Expiratory Pressure; MAP: mean arterial pressure; V-V ECMO: Veno-venous extracorporeal membrane oxygenation.
To calculate ORs, variables were dichotomized using the ICB group median before V-V ECMO as the cut-off point. The multivariable regression model included all factors with a p-value < 0.1 in the univariable analysis. According to the TRIPOD Statement, 18 assumed risk factors were not predefined in this exploratory analysis.
Akaike Information Criterion (AIC) was used to compare multivariable models. Lowest AIC is considered the best fitting model.
Delta values indicate the difference between pre-cannulation values and values 2 h after starting V-V ECMO. Extreme values indicate the patient’s highest or lowest value recorded until detection of ICB. One value is recorded daily.
p values below 0.05 were considered statistically significant. Statistical analyses were performed using IBM SPSS Statistic software version 25.0 (SPSS Inc. Chicago, IL, USA).
Impaired kidney function before ECMO was defined as a creatinine level higher than 1.5 mg/dl. KDIGO criteria defined acute kidney failure. Survival is defined as survival to hospital discharge.
Results
Patients
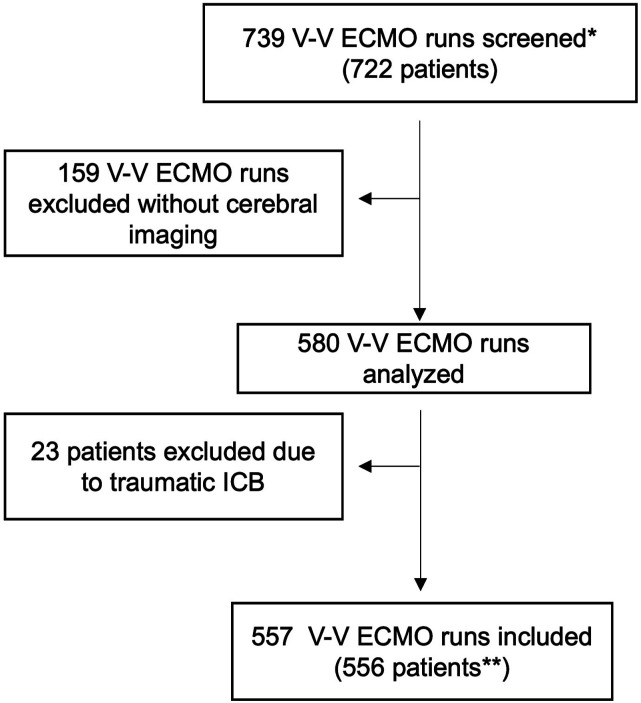
Among 722 patients (739 V-V ECMO runs) during the study period, 182 runs did not meet the inclusion criteria due to: (a) no cerebral imaging obtained during ECMO support (159 runs), and (b) cerebral bleeding diagnosed prior to ECMO cannulation or had ICB due to trauma (23 patients). In total, 556 patients were included in the analysis (Figure 1).
Figure 1.
Shows the process of inclusion in the analyses. Seven hundred thirty nine ECMO runs (722 patients were screened*). Five hundred and fifty six patients were included**. Exclusion of 182 patients due to no available cerebral imaging or ICB following trauma. Twenty of those without cerebral imaging were aged below 18 and had no CT due to avoidance of radiation exposure. The 139 remaining patients were treated with ECMO at the beginning of the observation period (n = 75) when CT scans during ECMO were performed less frequently at our institution, patients were considered too unstable for transport or had CT immediately prior to ECMO (n = 64).
Analysis of cerebral imaging and cerebral bleedings
In total, ICB was identified in 84 patients. In 23 patients ICB occurred after trauma, and these were excluded from the analysis. The ICBs included (n = 61, 61 patients) were detected by cCT (n = 54), MRI (n = 6) and by autopsy (n = 1). Only in 17 patients was ICB detected after clinical suspicion (disturbance in pupillomotorics, pupil difference, non-reaction to light n = 16, seizure n = 1), while 43 ICBs were detected by screening CT scans, and one bleeding was found by autopsy. The ICB localizations are shown in the Supplementary Table S1. Most patients showed more than one ICB localization.
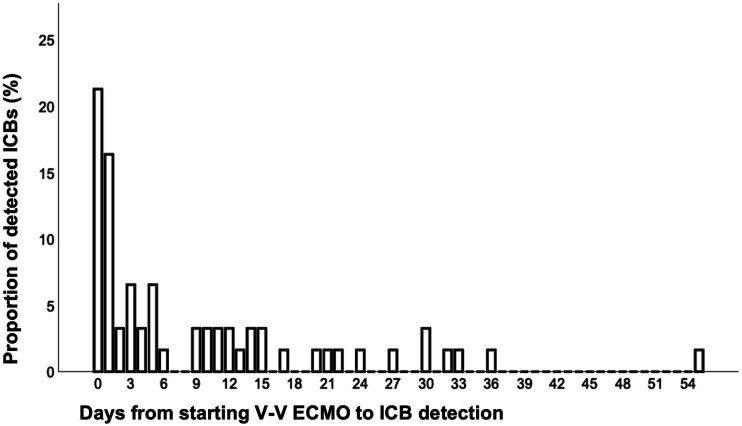
In median, ICB was detected 5 days (IQR 1-14) after starting V-V ECMO (Figure 2). Most ICBs (23/61; 37.7%) were detected on the day of V-V ECMO initiation or the day after.
Figure 2.
Shows the timing (days) of newly diagnosed intracranial bleedings (ICB) after V-V ECMO initiation as percentage of all detected bleeding events. The median time until ICB detection is 5 days (IQR 1-14 days). The latest ICB on day 55 after V-V ECMO was detected by MRI. Exact time of ICB recognition is stated in Supplementary Table S5. Most ICBs (23/61; 37.7%) were detected on the day of V-V ECMO initiation or the day after. Note: veno-venous extracorporeal membrane oxygenation.
In total, 7 ICBs were diagnosed after the end of V-V ECMO support (6 in MRI, 1 by autopsy), in median 27 days (IQR 21 – 41) after beginning V-V ECMO (median 18 days [IQR 9 – 35] after end of V-V ECMO support). Three of these patients died from multiorgan failure, four survived to hospital discharge.
The observed bleedings lead to an ICB frequency of 10.9% in our collective (61/556). If the frequency of subclinical ICB occurrence is taken as a basis, 12 subclinical ICBs were missed in the group of 159 patients who did not have cerebral imaging.
Baseline characteristics before V-V ECMO
Baseline characteristics and laboratory values before the start of V-V ECMO treatment are shown in Table 1. Additional baseline information is shown in Supplementary Table S2.
The survival to hospital discharge was 63.7% (n = 354) in all ECMO patients. The survival to discharge among patients suffering from ICB during V-V ECMO was 29.5%. Forty three patients with ICB died, 19 of them (44%) from ICB or from limiting therapy due to extensive ICB. Patients with ICB found incidentally (n = 44) had a survival of 36.4% (n = 16), which tended to be better compared to patients with clinical evidence of ICB (survival: 11.7%, p = 0.059). After adjustment for underlying disease severity (SOFA score), ICB was significantly associated with mortality (p < 0.001).
Among all survivors, CPC score at discharge was 1 in 84.5% (299/354), 2 in 14.4% (51/354) and 4 in 0.8% (3/354).
89% (16/18) of survivors with ICB had CPC score 1 at discharge, 11% (2/18) had CPC score 2 at hospital discharge.
93.7% (15/16) of survivors with ICB found incidentally had CPC 1 at discharge, 6.3% (1/16) had CPC score 2 at hospital discharge.
Among the group of patients developing ICB, laboratory parameters associated with coagulopathy and multiple organ failure were examined from the time of starting V-V ECMO to the diagnosis of ICB. For every single patient the variables were analyzed on the day before the bleeding diagnosis and during the course (Median) before recognition of bleeding (from starting V-V ECMO to the diagnosis of bleeding). The extreme value (highest or lowest) before ICB was obtained as well. The results were compared to the values before cannulation to show the course of the variables until ICB detection in patients developing ICB. Results are shown in Table 2.
Table 2.
Laboratory variables of patients suffering from ICB during V-V ECMO treatment. Laboratory parameters are shown before the start of ECMO, in median before recognition of ICB and the day before bleeding diagnosis, plus extreme values (highest or lowest) before bleeding. The variables were compared using the Wilcoxon-Test. Variables are shown as median and interquartile range (25th-75th).
| Variable | Pre-cannulation median (IQR 25th-75th) | Extreme value before ICB median (IQR 25th-75th) | p-value pre vs. Extreme value before ICB | Median before ICB median (IQR 25th-75th) | Day before ICB median (IQR 25th-75th) | p-value day before ICB vs. Pre-Cannulation |
|---|---|---|---|---|---|---|
| aPTT (s) | 46 (39.5–61.5) | 63 (50–105) | <0.001 | 49 (43–53) | 46 (40–57) | 0.659 |
| Fibrinogen (mg/dl) | 515 (352–646) | 367 (221–532) | <0.001 | 473 (333–611) | 435 (264–588) | 0.007 |
| Platelets (/nl) | 98 (47–212.5) | 75 (24–134) | <0.001 | 98 (37–176) | 98 (47–150) | 0.052 |
| Blood-urea (mg/dl) | 65 (47–130) | 100 (58–585) | <0.001 | 86 (54–123) | 89 (53–152) | 0.017 |
| Bilirubin (mg/dl) | 0.8 (0.6–2.4) | 2.6 (0.9–6.8) | <0.001 | 1.7 (0.7–4) | 1.6 (0.8–6.3) | <0.001 |
| ASAT (U/L) | 103 (43–208) | 128 (60–678) | <0.001 | 97 (46–240) | 89 (50–177) | 0.858 |
| LDH (U/L) | 550 (324–900) | 654 (428–1774) | <0.001 | 532 (376–910) | 510 (326–798) | 0.664 |
aPTT: activated partial Thromboplastin-Time; ASAT: Aspartat-Aminotransferasis; LDH: Lactate-Dehydrogenasis; V-V ECMO: Veno-venous extracorporeal membrane oxygenation.
Blood-urea and bilirubin are significantly higher during organ support with V-V ECMO and before bleeding than before ECMO. CRP is higher in the ICB group during ECMO support, but does not differ at the analyzed timepoints. The comparison of the variables during the clinical course between the different groups are shown in the Supplementary Table S2. A comparison of extreme values is shown in Supplementary Table S3.
Risk factors
To evaluate potential risk factors for developing ICB during V-V ECMO support, the Odds Ratios (OR) were calculated. The results are summarized in Table 3. Factors found in univariate analysis reflect severity of underlying disease (creatinine, blood-urea, LDH, norepinephrine), pre-existing coagulation disorders (aPTT, platelets) and rapid changes through ECMO initiation (change in paCO2 and change in norepinephrine). Factors with a p-value <0.1 in the univariate analysis were included in the multivariable analysis (creatinine aPTT, platelets, blood-urea, LDH, paCO2, norepinephrine, ASAT, SOFA score, pH). Multivariable analysis showed that low platelets, high paCO2 before ECMO, and rapid drop in paCO2 after ECMO are significant risk factors for ICB (Table 3 und Supplementary Table S6). SOFA score as an indicator of underlying disease severity 19 was not confirmed as an independent risk factor for developing ICB during V-V ECMO in the best model (Table 3).
Table 3.
Multivariable model of risk factors for developing ICB during V-V ECMO treatment. The tested variables were different between the groups before starting V-V ECMO, or additionally became different during ECMO treatment. Odds Ratios (OR) were calculated after dichotomizing the variables. The ICB group’s median was used as the cut-off value. Delta values indicate a difference between pre-cannulation values and values 2 h after starting V-V ECMO. CI95%: 95% confidence interval. Additional multivariable models, univariable calculations and number of patients at risk are summarized in Supplementary Table S6.
| Multivariable regression analysis model delta paCO2, delta norepinephrine, platelets <100/nl | |||
|---|---|---|---|
| Odds ratio | CI95% | p-value | |
| Creatinine >1.5mg/dl Before ECMO treatment |
1.09 | 0.53–2.34 | 0.812 |
| aPTT > 45s Before ECMO treatment |
1.09 | 0.57–2.08 | 0.793 |
| Platelets <100/nl Before ECMO treatment |
2.64 | 1.26–5.53 | 0.01 |
| Blood-urea >100 mg/dl Before ECMO treatment |
1.35 | 0.67–2.71 | 0.404 |
| LDH >550 U/L Before ECMO treatment |
1.60 | 0.82–3.11 | 0.170 |
| Delta paCO2 >35mmHg | 4.85 | 2.16–10.88 | <0.001 |
| Delta norepinephrine >1mg/h | 1.70 | 0.90–3.21 | 0.103 |
| ASAT > 80U/L Before ECMO treatment |
0.79 | 0.39–1.61 | 0.512 |
| SOFA score | 0.92 | 0.83–1.02 | 0.126 |
| pH before ECMO treatment | 0.03 | 0.001–0.745 | 0.032 |
SOFA: Sequential organ failure assessment; aPTT: activated partial Thromboplastin-Time; ASAT: Aspartat-Aminotransferasis; LDH: Lactate-Dehydrogenasis; V-V ECMO: Veno-venous extracorporeal membrane oxygenation.
Discussion
We identified ICB in 10.9% of patients treated with V-V ECMO. This is in accordance with the current literature reporting of ICB frequencies ranging from 2.5% up to 16.4%.6,12,13,16,20,21 A large retrospective register analysis of more than 7500 patients found a frequency of 4.5%. 6 However, radiological screening for ICB was not performed in previous studies,6,13 and therefore, the frequency of ICBs during V-V ECMO might be underestimated. In our study population, only 17 ICBs presented typical clinical symptoms, while the other 44 (72.1%) ICBs were detected without suggestive clinical symptoms, thus emphasizing the benefit of screening for ICB. If the frequency of subclinical ICB occurrence is taken as a basis, 12 subclinical ICBs were missed in the group of 159 patients who did not have cerebral imaging in our study. In our opinion, a routine cerebral CT is advisable within the first 3 days after V-V ECMO initiation, whenever chest or abdominal CT is performed, and whenever neurological abnormalities are found during clinical examination.
Survival among patients with ICB was lower (29.5%) than without ICB (67.9%), which is consistent with the current literature.6,12–14,22,23 Even after adjusting for underlying disease severity, ICB was a significant risk factor for mortality.
38% (23/61) of all bleeding events were detected the same day or the day after starting V-V ECMO, and median time to bleeding detection was 5 days, which is in line with other studies. 13 This emphasizes the importance of pre-ECMO conditions for developing ICB. Nevertheless, ICB during V-V ECMO may occur anytime while on ECMO, which contrasts with findings for V-A ECMO, 24 where almost all ICBs occur at the start of ECMO treatment. We cannot exclude, however, that ICB does in fact occur in even more cases within the first day after starting V-V ECMO but might be diagnosed later if the CT scan is performed later.
Potential risk factors reflecting coagulopathy and multiple organ failure did not differ, or just slightly differed, from pre-ECMO to ICB (Table 2). Therefore, in combination with the early occurrence of ICB, pre-ECMO conditions seem to be important risk factors for ICB. The risk factors for ICB found in our study can be divided into two groups. The first group reflects the severity of underlying disease, multiorgan failure and coagulation disorders, impaired kidney function, higher levels of blood-urea, aPTT, norepinephrine, LDH, paCO2, low platelets, elevated ASAT levels), which were also found in other studies as risk factors.13,14,17,25 Whether these factors contribute causally to ICB cannot be answered with our data. The second group reflects factors attributable to cannulation and ECMO initiation (change in paCO2 and norepinephrine). The association between decrease in paCO2 and ICB was found in other studies as well,13,15 and might be caused by paCO2’s contribution to cerebral vasotonus. 26 However, our study is the first showing an association between higher paCO2 levels before ECMO and ICB. Our results revealed thrombocytopenia and disturbance in paCO2 as independent risk factors (Table 3), whereas SOFA score as an indicator of underlying disease severity 19 was not confirmed as an independent risk factor for developing ICB during V-V ECMO (Table 3).
Due to the poor survival of patients developing ICB, preventing ICB is important. Risk factors existing prior to ECMO might help to identify patients at risk for ICB. Although the data shown here are retrospective, a slow decrease in paCO2 is recommended as this can be easily controlled. Prospective data on the speed of lowering are lacking. In our center, for hemodynamic stability the normalization of paCO2 over at least 2 h is the goal. However, it remains unclear whether ECMO or underlying disease 17 is crucial for developing ICB. Probably, ECMO and its influence on platelets27,28 and blood-gases as well as hemodynamic instability pre-ECMO result in a predisposition towards ICB.
The strength of our study analyses is the large cohort of patients (n = 556) and screening for ICB even without neurological symptoms. In this respect, our data differs from the existing literature, and may provide better evidence about the frequency of ICB in a V-V ECMO cohort.
Limitations
This study has several limitations. First, it is a retrospective monocentric study. Second, screening was performed, but no systematic or standardized protocol was used, therefore it cannot be ruled out that ICB may have already occurred in many cases before cannulation since no cerebral imaging was performed immediately before and after cannulation, and thus the time of ICB might be biased. Furthermore, 159 ECMO runs, especially at the beginning of the study period, were excluded due to missing cerebral imaging. Thus, the frequency of ICB observed in our trial is biased because the frequency of ICB in the group of patients without cerebral imaging may be different.
Conclusion
Due to the high number (72.1%) of subclinical ICBs, a low threshold for performing cerebral imaging during V-V ECMO, especially at the beginning of V-V ECMO support, is reasonable and advisable. The risk factors for developing ICB found in this analysis mainly existed prior to initiating V-V ECMO, and either reflect underlying disease severity or develop within the first few hours. These factors could help identify patients at risk for developing ICB. Independent risk factors for developing ICB were higher paCO2 and lower platelets before V-V ECMO. Therefore rapid decrease in paCO2 should be avoided.
Supplemental Material
Supplemental Material for Intracranial hemorrhage in a large cohort of patients supported with veno-venous ECMO. A retrospective single-center analysis by Clemens Wiest, Thomas Müller, Matthias Lubnow, Christoph Fisser, Alois Philipp, Maik Foltan, Roland Schneckenpointner and Maximilian V Malfertheiner in Perfusion
Acknowledgements
We thank the nursing staff and doctors of the ICUs at the University Hospital Regensburg for their excellent patient care, and, in particular, all perfusionists for the daily technical support and meticulous maintenance of the database. We thank Dr. Florian Poschenrieder for radiological support in CT scan evaluation and Florian Zeman for statistical advice and support.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Supplemental Material: Supplemental material for this article is available online.
Ethical statement
Ethical Approval
This study has been approved by the University Regensburg Ethics Committee (number 22-2770-104).
ORCID iDs
Clemens Wiest https://orcid.org/0000-0002-9735-0781
Maik Foltan https://orcid.org/0000-0001-9350-3368
References
- 1.Makdisi G, Wang IW. Extra corporeal membrane oxygenation (ECMO) review of a lifesaving technology. J Thorac Dis 2015; 7(7): E166–E176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friedrichson B, Mutlak H, Zacharowski K, et al. Insight into ECMO, mortality and ARDS: a nationwide analysis of 45,647 ECMO runs. Crit Care 2021; 25(1): 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thiagarajan RR, Barbaro RP, Rycus PT, et al. Extracorporeal life support organization registry international report 2016. Asaio J 2017; 63(1): 60–67. [DOI] [PubMed] [Google Scholar]
- 4.Tonna JE, Abrams D, Brodie D, et al. Management of adult patients supported with venovenous extracorporeal membrane oxygenation (VV ECMO): guideline from the extracorporeal life support organization (ELSO). Asaio J 2021; 67(6): 601–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fisser C, Reichenbächer C, Müller T, et al. Incidence and risk factors for cannula-related venous thrombosis after venovenous extracorporeal membrane oxygenation in adult patients with acute respiratory failure. Crit Care Med 2019; 47(4): e332–e339. [DOI] [PubMed] [Google Scholar]
- 6.Nunez JI, Gosling AF, O'Gara B, et al. Bleeding and thrombotic events in adults supported with venovenous extracorporeal membrane oxygenation: an ELSO registry analysis. Intensive Care Med 2022; 48(2): 213–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cavayas YA, del Sorbo L, Fan E. Intracranial hemorrhage in adults on ECMO. Perfusion 2018; 33(1_suppl): 42–50. [DOI] [PubMed] [Google Scholar]
- 8.Aubron C, DePuydt J, Belon F, et al. Predictive factors of bleeding events in adults undergoing extracorporeal membrane oxygenation. Ann Intensive Care 2016; 6(1): 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lan C, Tsai PR, Chen YS, et al. Prognostic factors for adult patients receiving extracorporeal membrane oxygenation as mechanical circulatory support--a 14-year experience at a medical center. Artif Organs 2010; 34(2): E59–E64. [DOI] [PubMed] [Google Scholar]
- 10.Carod-Artal FJ, Egido JA. Quality of life after stroke: the importance of a good recovery. Cerebrovasc Dis 2009; 27(Suppl 1): 204–214. [DOI] [PubMed] [Google Scholar]
- 11.von Bahr V, Kalzén H, Hultman J, et al. Long-term cognitive outcome and brain imaging in adults after extracorporeal membrane oxygenation. Crit Care Med 2018; 46(5): e351–e358. [DOI] [PubMed] [Google Scholar]
- 12.Lorusso R, Gelsomino S, Parise O, et al. Neurologic injury in adults supported with veno-venous extracorporeal membrane oxygenation for respiratory failure: findings from the extracorporeal life support organization database. Crit Care Med 2017; 45(8): 1389–1397. [DOI] [PubMed] [Google Scholar]
- 13.Luyt CE, Brechot N, Demondion P, et al. Brain injury during venovenous extracorporeal membrane oxygenation. Intensive Care Med 2016; 42(5): 897–907. [DOI] [PubMed] [Google Scholar]
- 14.Fletcher-Sandersjoo A, Thelin EP, Bartek J,, Jr., et al. Incidence, outcome, and predictors of intracranial hemorrhage in adult patients on extracorporeal membrane oxygenation: a systematic and narrative review. Front Neurol 2018; 9: 548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cavayas YA, Munshi L, Del Sorbo L, et al. The early change in Pa(CO(2)) after extracorporeal membrane oxygenation initiation is associated with neurological complications. Am J Respir Crit Care Med 2020; 201(12): 1525–1535. [DOI] [PubMed] [Google Scholar]
- 16.Lockie CJA, Gillon SA, Barrett NA, et al. Severe respiratory failure, extracorporeal membrane oxygenation, and intracranial hemorrhage. Crit Care Med 2017; 45(10): 1642–1649. [DOI] [PubMed] [Google Scholar]
- 17.Arachchillage DRJ, Passariello M, Laffan M, et al. Intracranial hemorrhage and early mortality in patients receiving extracorporeal membrane oxygenation for severe respiratory failure. Semin Thromb Hemost 2018; 44(3): 276–286. [DOI] [PubMed] [Google Scholar]
- 18.Collins GS, Reitsma JB, Altman DG, et al. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. BMJ 2015; 350: g7594. [DOI] [PubMed] [Google Scholar]
- 19.Hsin CH, Wu MY, Huang CC, et al. Venovenous extracorporeal membrane oxygenation in adult respiratory failure: Scores for mortality prediction. Medicine 2016; 95(25): e3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Combes A, Hajage D, Capellier G, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med 2018; 378(21): 1965–1975. [DOI] [PubMed] [Google Scholar]
- 21.Shoskes A, Migdady I, Rice C, et al. Brain injury is more common in venoarterial extracorporeal membrane oxygenation than venovenous extracorporeal membrane oxygenation: a systematic review and meta-analysis. Crit Care Med 2020; 48(12): 1799–1808. [DOI] [PubMed] [Google Scholar]
- 22.Davies A, Jones D, Bailey M, et al. Extracorporeal membrane oxygenation for 2009 influenza A(H1N1) acute respiratory distress syndrome. JAMA 2009; 302(17): 1888–1895. [DOI] [PubMed] [Google Scholar]
- 23.Patroniti N, Zangrillo A, Pappalardo F, et al. The Italian ECMO network experience during the 2009 influenza A(H1N1) pandemic: preparation for severe respiratory emergency outbreaks. Intensive Care Med 2011; 37(9): 1447–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malfertheiner MV, Koch A, Fisser C, et al. Incidence of early intra-cranial bleeding and ischaemia in adult veno-arterial extracorporeal membrane oxygenation and extracorporeal cardiopulmonary resuscitation patients: a retrospective analysis of risk factors. Perfusion 2020; 35(1_suppl): 8–17. [DOI] [PubMed] [Google Scholar]
- 25.Fletcher-Sandersjoo A, Thelin EP, Bartek J,, Jr., et al. Management of intracranial hemorrhage in adult patients on extracorporeal membrane oxygenation (ECMO): An observational cohort study. PLoS One 2017; 12(12): e0190365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kredel M, Lubnow M, Westermaier T, et al. Cerebral tissue oxygenation during the initiation of venovenous ECMO. Am Soc Artif Intern Organs J 2014; 60(6): 694–700. [DOI] [PubMed] [Google Scholar]
- 27.Balle CM, Jeppesen AN, Christensen S, et al. Platelet function during extracorporeal membrane oxygenation in adult patients. Front Cardiovasc Med 2019; 6: 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiritano F, Serraino GF, Ten Cate H, et al. Platelets and extra-corporeal membrane oxygenation in adult patients: a systematic review and meta-analysis. Intensive Care Med 2020; 46(6): 1154–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Intracranial hemorrhage in a large cohort of patients supported with veno-venous ECMO. A retrospective single-center analysis by Clemens Wiest, Thomas Müller, Matthias Lubnow, Christoph Fisser, Alois Philipp, Maik Foltan, Roland Schneckenpointner and Maximilian V Malfertheiner in Perfusion