Table 1.
Clinical trials of different FLT3 inhibitors including the FDA approved TKIs (midostaurin and gilteritinib)a.
| Drug | aChemical structure | Clinical trial ID | Sample size (year age) | Intervention/Treatment | Efficacy | Phase | Ref. |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Midostaurin |
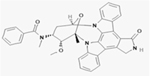
|
NCT00651261 | 717 (18–59) | Cytarabine and daunorubicin plus, midostaurin versus placebo in newly diagnosed AML patients | Median OS: 74.7 months, Midostaurin group; 25.6 months, Placebo group Median EFS: 8.2 months (95% CI, 5.4 to 10.7), midostaurin group; 3.0 months (95% CI, 1.9 to 5.9), placebo group | III | (Stone et al., 2017) |
| Gilteritinib (ASP2215) |
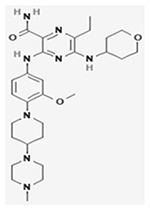
|
NCT02421939 | 371(18 and older) | Gilteritinib (ASP2215) versus salvage chemotherapy in R/R AML patients harboring FLT3 mutation | Median OS: 9.3 months, Gilteritinib; 5.6 months, Chemotherapy group Median EFS: 2.8 months, gilteritinib group; 0.7 months, chemotherapy group | III | (Perl et al., 2019) |
| Sorafenib |
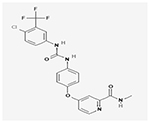
|
NCT00893373 | 276 (18–60) |
Sorafenib added to standard primary therapy in newly diagnosed AML patients | median EFS 9 months, placebo group versus 21 months, sorafenib group; 3-year-EFS of 22%, placebo group versus 40%, sorafenib group | II | (Rollig et al., 2015) |
| Lestaurtinib (CEP-701) |
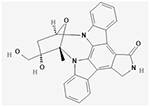
|
NCT00079482; | 224(18 and older); | Lestaurtinib (CEP-701) given in sequence with induction chemotherapy | 29 patients with CR/CRp in the lestaurtinib arm and 23 in the control arm; No difference in OS between the 2 arms; No overall clinical benefit | II | (Levis et al., 2011) |
| ISRCTN17161961and | (Knapper et al., 2017) | ||||||
| ISRCTN55675535 | 500(5 to 68) | in patients with relapsed AML expressing FLT3 activating mutations; patients with newly diagnosed AML or high-risk myelodysplastic syndrome | III | (Knapper et al., 2017) | |||
| Quizartinib |
|
NCT02039726 | 367(18 and older) | Quizartinib Monotherapy vs. Salvage Chemotherapy | Median OS: 6·2 months (5·3–7·2), quizartinib group; 4·7 months (4·0–5·5), chemotherapy group | III | (Cortes et al., 2019) |
References of 2D structures in Table 1.
1. PubChem Identifier: CID 126565 (Lestaurtinib)
2. PubChem Identifier: CID 216239 (Sorafenib)
3. PubChem Identifier: CID 24889392 (Quizartinib)
4. PubChem Identifier: CID 49803313 (Gilteritinib)
6. PubChem Identifier: CID 9829523 (Midostaurin)
