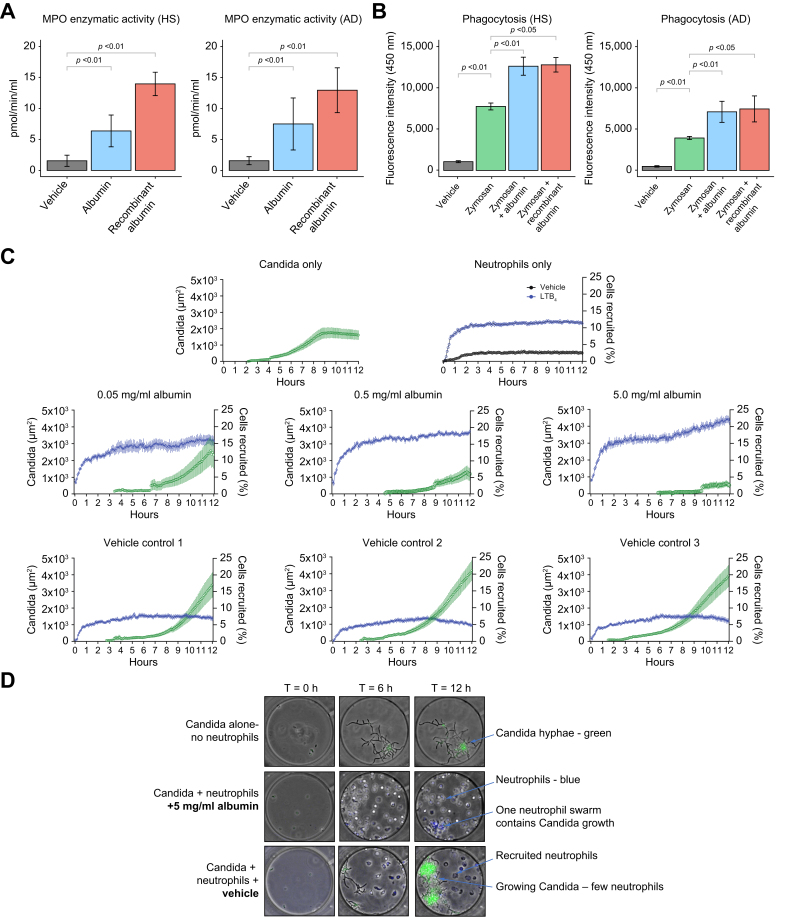
Fig. 4.
Effects of HSA on the host defense function of neutrophils from patients with AD cirrhosis.
(A) Neutrophils were incubated with cell medium (vehicle), HSA, or recombinant human albumin (both at 15 mg/ml) for 2 h at 37 °C in a 5% CO2 incubator. Neutrophil degranulation was assessed by measuring the MPO enzymatic activity in the cell supernatants. (B) Phagocytic capacity assessed by incubating neutrophils with FITC-conjugated zymosan bioparticles alone or in the presence of HSA and recombinant albumin for 60 min and compared with vehicle control. (C) Quantification of Candida albicans growth and neutrophil recruitment in the microfluidic device. The size of Candida hyphae clusters was quantified based on the area of green fluorescence and is shown in green. The number of neutrophils recruited was quantified and shown in blue. For the neutrophils-only condition, we compared the neutrophils entering the chambers in the presence of the vehicle (black) with the number of neutrophils in the presence of LTB4 chemoattractant (100 nM; blue). Twelve chambers per condition were quantified, and three of them are shown. (D) Microscopy images of neutrophil–Candida albicans interactions in microfluidic chambers. A similar number of Candida yeast was loaded in each chamber (time T-0). Neutrophils were loaded outside the chambers. Neutrophils migrated to the chambers, attracted by Candida-released molecules. In the presence of HSA, neutrophils actively phagocytosed Candida. Neutrophils swarmed around any Candida hyphae clusters, delaying their growth. In vehicle controls, neutrophils delayed the growth of Candida but were not efficient enough to contain clusters of Candida hyphae. (A)–(D) were designed using functional assays in freshly isolated peripheral neutrophils from six patients with AD cirrhosis and five age-matched HV. Significant differences between groups were assessed using paired t tests. AD, acutely decompensated; HSA, human serum albumin; HV, healthy volunteers; LTB4, leukotriene B4; MPO, myeloperoxidase.