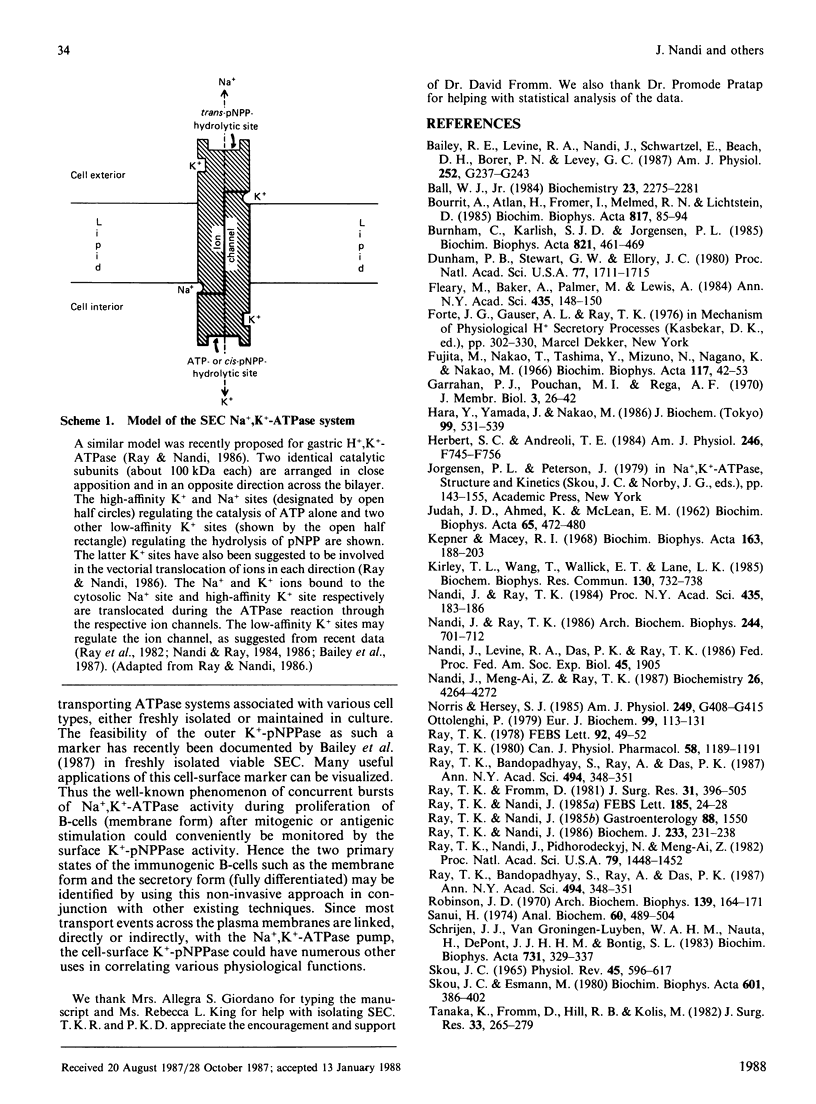
Abstract
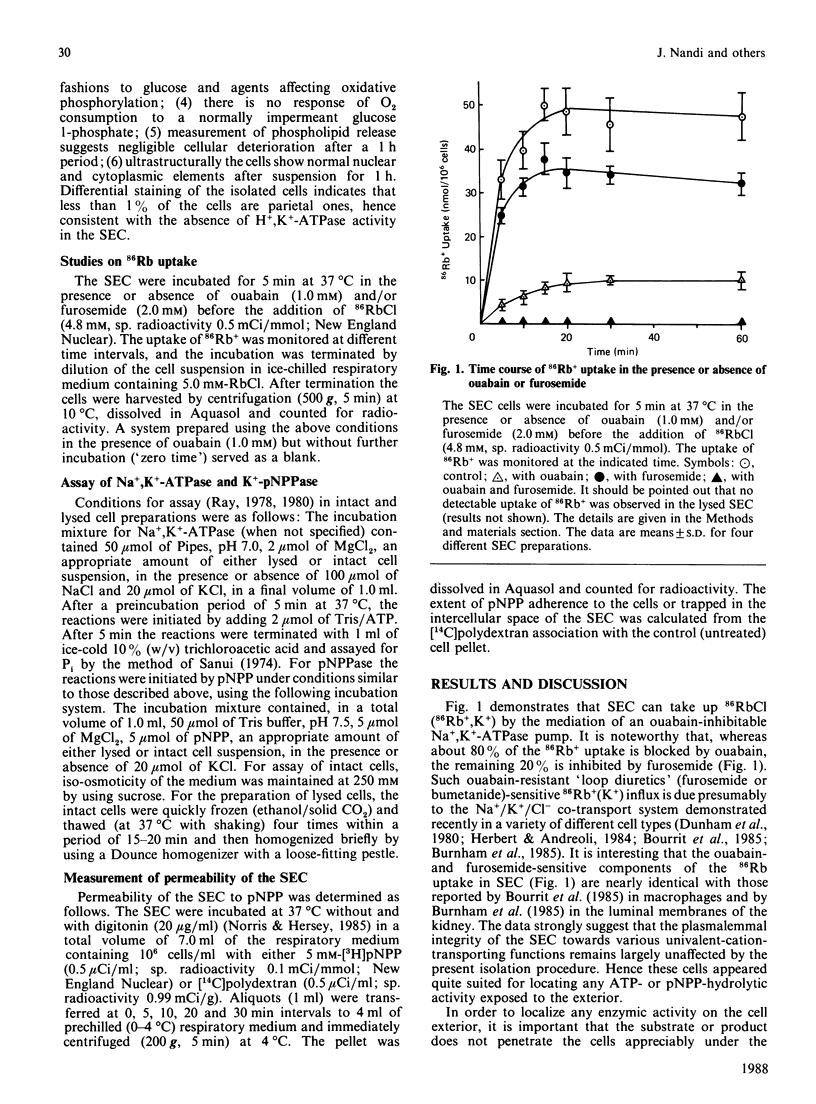
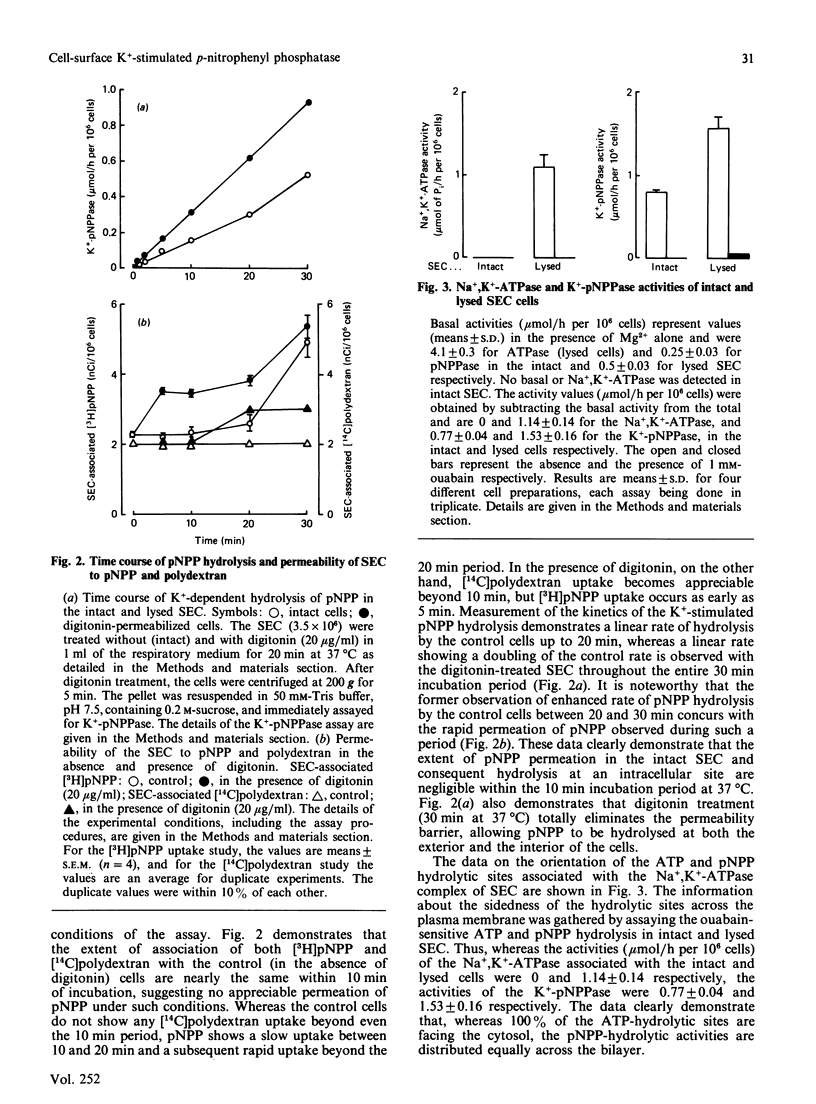
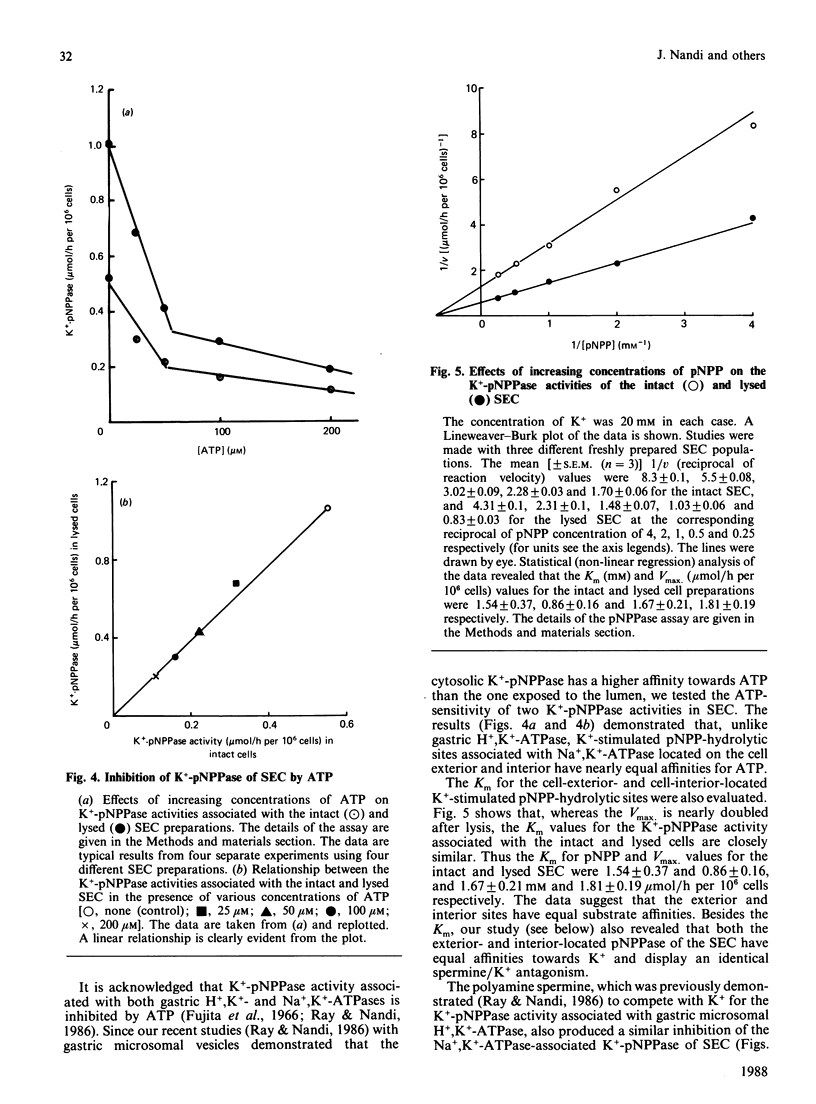
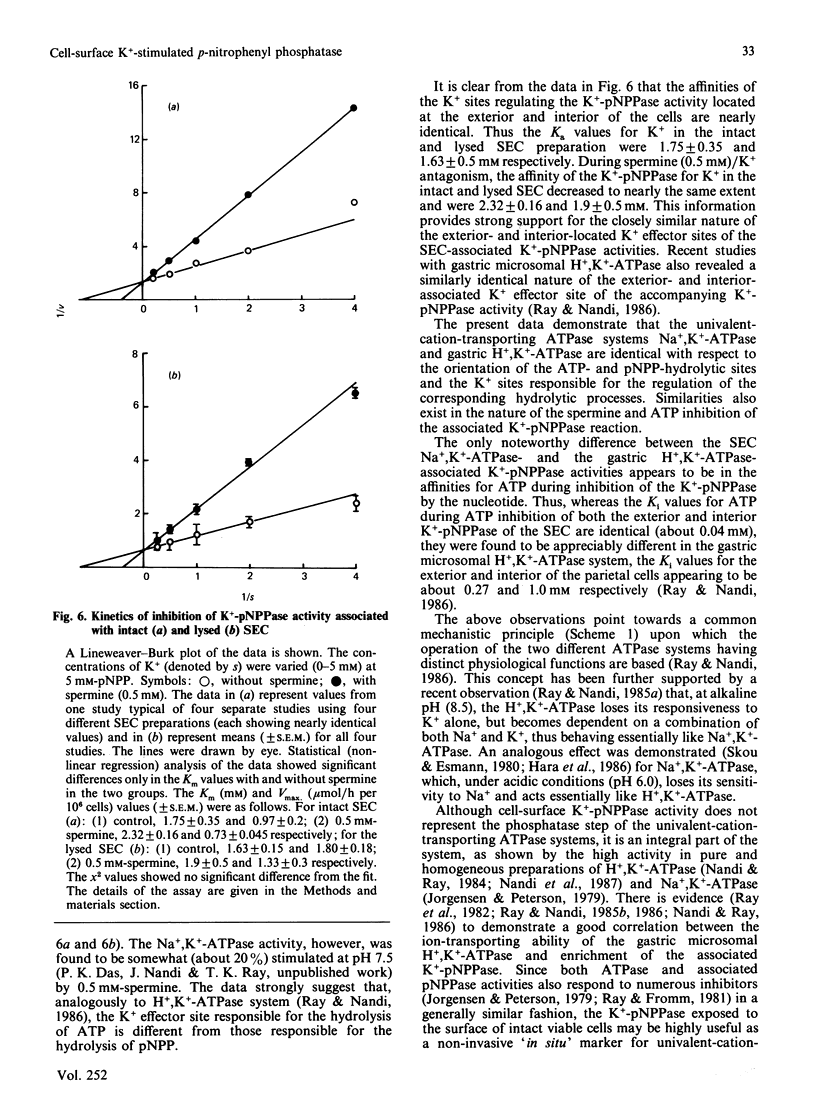
Ouabain inhibited 86RbCl uptake by 80% in rabbit gastric superficial epithelial cells (SEC), revealing the presence of a functional Na+,K+-ATPase [(Na+ + K+)-transporting ATPase] pump. Intact SEC were used to study the ouabain-sensitive Na+,K+-ATPase and K+-pNPPase (K+-stimulated p-nitrophenyl phosphatase) activities before and after lysis. Intact SEC showed no Na+,K+-ATPase and insignificant Mg2+-ATPase activity. However, appreciable K+-pNPPase activity sensitive to ouabain inhibition was demonstrated by localizing its activity to the cell-surface exterior. The lysed SEC, on the other hand, demonstrated both ouabain-sensitive Na+,K+-ATPase and K+-pNPPase activities. Thus the ATP-hydrolytic site of Na+,K+-ATPase faces exclusively the cytosol, whereas the associated K+-pNPPase is distributed equally across the plasma membrane. The study suggests that the cell-exterior-located K+-pNPPase can be used as a convenient and reliable 'in situ' marker for the functional Na+,K+-ATPase system of various isolated cells under noninvasive conditions.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey R. E., Levine R. A., Nandi J., Schwartzel E. H., Jr, Beach D. H., Borer P. N., Levy G. C. Effects of ethanol on gastric epithelial cell phospholipid dynamics and cellular function. Am J Physiol. 1987 Feb;252(2 Pt 1):G237–G243. doi: 10.1152/ajpgi.1987.252.2.G237. [DOI] [PubMed] [Google Scholar]
- Ball W. J., Jr Immunochemical characterization of a functional site of (Na+,K+)-ATPase. Biochemistry. 1984 May 8;23(10):2275–2281. doi: 10.1021/bi00305a029. [DOI] [PubMed] [Google Scholar]
- Bourrit A., Atlan H., Fromer I., Melmed R. N., Lichtstein D. Basic characterization of an ouabain-resistant, bumetanide-sensitive K+ carrier-mediated transport system in J774.2 mouse macrophage-like cell line and in variants deficient in adenylate cyclase and cAMP-dependent protein kinase activities. Biochim Biophys Acta. 1985 Jul 11;817(1):85–94. doi: 10.1016/0005-2736(85)90071-9. [DOI] [PubMed] [Google Scholar]
- Burnham C., Karlish S. J., Jørgensen P. L. Identification and reconstitution of a Na+/K+/Cl- cotransporter and K+ channel from luminal membranes of renal red outer medulla. Biochim Biophys Acta. 1985 Dec 19;821(3):461–469. doi: 10.1016/0005-2736(85)90051-3. [DOI] [PubMed] [Google Scholar]
- Dunham P. B., Stewart G. W., Ellory J. C. Chloride-activated passive potassium transport in human erythrocytes. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1711–1715. doi: 10.1073/pnas.77.3.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita M., Nakao T., Tashima Y., Mizuno N., Nagano K., Nakao M. Potassium-ion stimulated p-nitrophenylphosphatase activity occurring in a highly specific adenosine triphosphatase preparation from rabbit brain. Biochim Biophys Acta. 1966 Mar 28;117(1):42–53. doi: 10.1016/0304-4165(66)90150-4. [DOI] [PubMed] [Google Scholar]
- Hara Y., Yamada J., Nakao M. Proton transport catalyzed by the sodium pump. Ouabain-sensitive ATPase activity and the phosphorylation of Na,K-ATPase in the absence of sodium ions. J Biochem. 1986 Feb;99(2):531–539. doi: 10.1093/oxfordjournals.jbchem.a135509. [DOI] [PubMed] [Google Scholar]
- Hebert S. C., Andreoli T. E. Control of NaCl transport in the thick ascending limb. Am J Physiol. 1984 Jun;246(6 Pt 2):F745–F756. doi: 10.1152/ajprenal.1984.246.6.F745. [DOI] [PubMed] [Google Scholar]
- Kepner G. R., Macey R. I. Membrane enzyme systems. Molecular size determinations by radiation inactivation. Biochim Biophys Acta. 1968 Sep 17;163(2):188–203. doi: 10.1016/0005-2736(68)90097-7. [DOI] [PubMed] [Google Scholar]
- Kirley T. L., Wang T., Wallick E. T., Lane L. K. Homology of ATP binding sites from Ca2+ and (Na,K)-ATPases: comparison of the amino acid sequences of fluorescein isothiocyanate labeled peptides. Biochem Biophys Res Commun. 1985 Jul 31;130(2):732–738. doi: 10.1016/0006-291x(85)90477-2. [DOI] [PubMed] [Google Scholar]
- Nandi J., Ray T. K. Mechanism of gastric antisecretory effect of thiocyanate: further evidence for the thiocyanate-induced impediment in gastric H+,K+-ATPase function. Arch Biochem Biophys. 1986 Feb 1;244(2):701–712. doi: 10.1016/0003-9861(86)90639-9. [DOI] [PubMed] [Google Scholar]
- Nandi J., Zhou M. A., Ray T. K. Purification and partial characterization of the (H+,K+)-transporting adenosinetriphosphatase from fundic mucosa. Biochemistry. 1987 Jul 14;26(14):4264–4272. doi: 10.1021/bi00388a013. [DOI] [PubMed] [Google Scholar]
- Norris S. H., Hersey S. J. Stimulation of pepsinogen secretion in permeable isolated gastric glands. Am J Physiol. 1985 Sep;249(3 Pt 1):G408–G415. doi: 10.1152/ajpgi.1985.249.3.G408. [DOI] [PubMed] [Google Scholar]
- Ottolenghi P. The relipidation of delipidated Na,K-ATPase. An analysis of complex formation with dioleoylphosphatidylcholine and with dioleoylphosphatidylethanolamine. Eur J Biochem. 1979 Aug 15;99(1):113–131. doi: 10.1111/j.1432-1033.1979.tb13238.x. [DOI] [PubMed] [Google Scholar]
- Ray T. K. Effects of barium on gastric microsomal K+-stimulated para-nitrophenyl phosphatase activity. Can J Physiol Pharmacol. 1980 Oct;58(10):1189–1191. doi: 10.1139/y80-181. [DOI] [PubMed] [Google Scholar]
- Ray T. K., Fromm D. Cellular and subcellular aspects of the mechanism of gastric acid secretion. J Surg Res. 1981 Dec;31(6):496–505. doi: 10.1016/0022-4804(81)90188-8. [DOI] [PubMed] [Google Scholar]
- Ray T. K. Gastric K+-stimulated adenosine triphosphatase. Demonstration of an endogenous activator. FEBS Lett. 1978 Aug 1;92(1):49–52. doi: 10.1016/0014-5793(78)80719-4. [DOI] [PubMed] [Google Scholar]
- Ray T. K., Nandi J. K+-stimulated p-nitrophenyl phosphatase is not a partial reaction of the gastric (H+ + K+)-transporting ATPase. Evidence supporting a new model for the univalent-cation-transporting ATPase systems. Biochem J. 1986 Jan 1;233(1):231–238. doi: 10.1042/bj2330231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray T. K., Nandi J. Modulation of gastric H+,K+-transporting ATPase function by sodium. FEBS Lett. 1985 Jun 3;185(1):24–28. doi: 10.1016/0014-5793(85)80733-x. [DOI] [PubMed] [Google Scholar]
- Ray T. K., Nandi J., Pidhorodeckyj N., Meng-Ai Z. Polyamines are inhibitors of gastric acid secretion. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1448–1452. doi: 10.1073/pnas.79.5.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J. D. Phosphatase activity stimulated by Na+ plus K+: implications for the (Na+ plus K+)-dependent adenosine triphosphatase. Arch Biochem Biophys. 1970 Jul;139(1):164–171. doi: 10.1016/0003-9861(70)90057-3. [DOI] [PubMed] [Google Scholar]
- SKOU J. C. ENZYMATIC BASIS FOR ACTIVE TRANSPORT OF NA+ AND K+ ACROSS CELL MEMBRANE. Physiol Rev. 1965 Jul;45:596–617. doi: 10.1152/physrev.1965.45.3.596. [DOI] [PubMed] [Google Scholar]
- Sanui H. Measurement of inorganic orthophosphate in biological materials: extraction properties of butyl acetate. Anal Biochem. 1974 Aug;60(2):489–504. doi: 10.1016/0003-2697(74)90259-0. [DOI] [PubMed] [Google Scholar]
- Schrijen J. J., Van Groningen-Luyben W. A., Nauta H., De Pont J. J., Bonting S. L. Studies on (K+ + H+)-ATPase. VI. Determination on the molecular size by radiation inactivation analysis. Biochim Biophys Acta. 1983 Jun 10;731(2):329–337. doi: 10.1016/0005-2736(83)90025-1. [DOI] [PubMed] [Google Scholar]
- Skou J. C., Esmann M. Effects of ATP and protons on the Na : K selectivity of the (Na+ + K+)-ATPase studied by ligand effects on intrinsic and extrinsic fluorescence. Biochim Biophys Acta. 1980 Sep 18;601(2):386–402. doi: 10.1016/0005-2736(80)90543-x. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Fromm D., Hill R. B., Kolis M. Isolation and viability of gastric mucosal surface cells of the rabbit. J Surg Res. 1982 Oct;33(4):265–279. doi: 10.1016/0022-4804(82)90039-7. [DOI] [PubMed] [Google Scholar]


