Abstract
Background
Infection remains a significant concern following penile prosthesis (PP) implantation surgery. Published guidelines have indicated the use of pre-operative intravenous (IV) antibiotics but have not provided specific recommendations regarding intra-operative irrigation. For long in our practice, we have been using a combination of antibiotics for irrigation (rifampin 600 mg/L of sterile water, gentamicin 80 mg/L of sterile water). Recently, 0.05% chlorhexidine gluconate (CHG) (Irrisept®) (Irrimax Corp, Lawrenceville, GA, USA), has shown promise as an alternative irrigant, with potential advantages in terms of cost, ease of administration and reduced antibiotic resistance risk. The study aims to assess the non-inferiority of CHG antiseptic irrigation compared to conventional combined antibiotic irrigation in preventing postoperative infections for men undergoing de-novo PP implantation.
Methods
This is a two-center Institutional Review Board (IRB) approved prospective randomized controlled non inferiority trial, involving men undergoing de-novo PP implantation with a predetermined non-inferiority margin of 4%. Patients were randomly assigned into one of two groups: the CHG irrigation or the group receiving conventional antibiotic irrigation. All patients received IV antibiotics preoperatively and were sent home on oral antibiotics for 2 weeks post-operatively. The primary endpoint of this trial was to evaluate the incidence of PP infections. Secondary objectives encompassed the assessment of simplicity of use and cost-effectiveness.
Results
A hundred patients were enrolled in our study so far (50 in each arm). Only one case of PP infection necessitating explanation was reported in each arm. Moreover, the use of CHG irrigation offered the potential advantages of ease of administration and less cost as compared to antibiotic irrigation solutions.
Conclusions
Preliminary data from this non-inferiority study demonstrate that CHG irrigation is non-inferior to conventional antibiotic irrigation in preventing postoperative infection following PP implantation. These findings support the consideration of CHG irrigation as a viable alternative during PP implantation, offering both clinical effectiveness and potential cost savings as well as reducing the risk of antibiotic resistance.
Trial Registration
ClinicalTrials.gov NCT06489431.
Keywords: Penile prosthesis (PP), infection, antiseptic, irrigation
Introduction
Infection remains a significant concern following penile prosthesis (PP) implantation surgery that can jeopardize the success of the implant, patient well-being, and overall healthcare costs. Historical rates of infection have been as high as 5% in first-time implants in experienced hands but PP device innovations such as infection retardant coating and adaptations in surgical technique have led to device infection rates between 1–3% among high volume implanters in modern series (1-5).
The pattern of organisms causing infections after penile implant procedures is evolving, moving away from predominantly less virulent gram-positive infections and toward more virulent gram-negative and fungal infections. This change can be linked to the selective pressure on antibiotics (6-8). While guidelines provide recommendations for preoperative systemic antibiotics, they do not provide specific guidance as regards the choice of intra-operative irrigation solutions (9).
Intra-operative irrigation during PP implantation surgery has traditionally involved the use of a combination of antibiotics such as Rifampin and Gentamicin, covering most Gram positive and negative bacteria (5). While this practice has been effective in many cases, it is not without its limitations. One of the significant drawbacks of conventional antibiotic irrigation is the potential for the development of antibiotic resistance. Additionally, the process of preparing the irrigation solution as well as the cost, are relevant factors to be considered (10).
Recently, a novel alternative to conventional antibiotic irrigation has emerged in the form of a solution containing 0.05% chlorhexidine gluconate (CHG) (Irrisept®) (Irrimax Corp, Lawrenceville, Georgia, USA), that is a broad-spectrum antiseptic with a well-established track record in infection control (11,12). Research has demonstrated 0.05% CHG as a highly effective antibacterial, antifungal and antiviral agent. It achieves this by binding to anionic molecules in the cell wall causing its disruption, resulting in a significant reduction in microbial presence. Moreover, 0.05% CHG exhibits minimal tissue reactivity. As a result, CHG has gained popularity among prosthetic urologists and is increasingly being integrated into their implant surgeries (11-13).
This study aims to address the existing knowledge gap by systematically evaluating the non-inferiority of CHG in comparison to the conventional combined antibiotic irrigation approach. It focuses on its ability to prevent postoperative infections, potentially advancing the standard of care for PP implantation procedures by offering a simpler, more cost-effective, and less resistance-prone approach to intra-operative irrigation. We present this article in accordance with the CONSORT reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-24-278/rc).
Methods
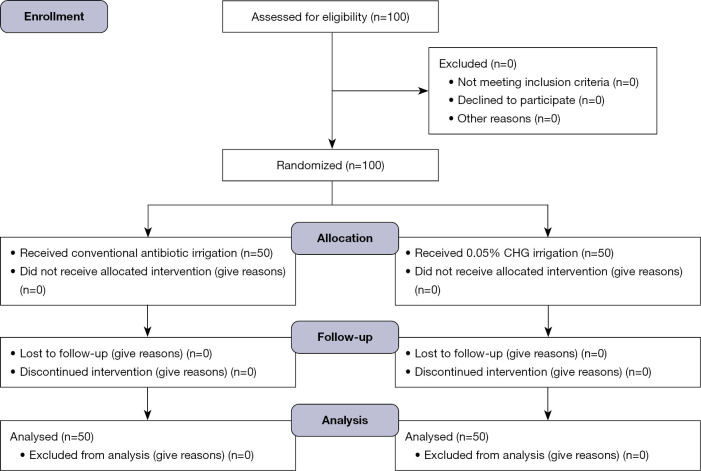
This is a two-center Institutional Review Board (IRB) approved prospective randomized controlled non-inferiority study, involving men undergoing de-novo PP implantation conducted by 2 primary surgeons at 2 medical facilities (Rush University Medical Center & Uropartners Solaris Health, Chicago, IL, USA) (Figure 1).
Figure 1.
Flow Diagram of results of our study comparing chlorhexidine gluconate (CHG) versus conventional antibiotic irrigation for de-novo penile prosthesis implantation.
The study was approved by Institutional Review Board of Rush University Medical Center (Approval No. 21120903-IRB01). The other institution (Uropartners Solaris Health) was informed and agreed with the study. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was taken from all participants. Patients were eligible for enrollment if they met the following criteria:
❖ Age 18 to 70 years;
❖ Diagnosis of erectile dysfunction requiring PP implantation;
❖ No active infection at the time of surgery;
❖ If the patient is diabetic, HbA1c has to be less than 8%;
❖ Willingness to participate and provide informed consent.
We included patients who underwent simple straightforward additional procedures such as circumcision, manual modeling for Peyronie’s disease (PD) curvature, or vasectomy in our cohort.
Exclusion criteria:
❖ Redo inflatable penile prosthesis (IPP) cases;
❖ Extensive additional procedure e.g., excision and grafting or tunica expansion procedures.
Patients were sequentially randomized into one of two study groups and surgeons were not blinded to the type of irrigation:
❖ Chlorhexidine group: patients in this group received 0.05% CHG irrigation during the PP implantation surgery. General scrotal wound and implant irrigation were performed throughout the procedure. Following the manufacturer’s recommendation, the Titan® device (Coloplast, Minneapolis, MN, USA) underwent both, the irrigation process and dipping during device preparation on the back table, unlike the CX700® (Boston Scientific, Minneapolis, MN, USA), which did not undergo dipping.
❖ Conventional antibiotic group: patients in this group received conventional antibiotic irrigation with two separate solutions (rifampin 600 mg/L of sterile water, gentamicin 80 mg/L of sterile water) during the surgery.
All patients received pre-operative intravenous antibiotics as per established guidelines. The choice of antibiotics was consistent across both study groups (vancomycin 15 mg/kg, gentamicin 7 mg/kg +/− fluconazole 200 mg in case the patient is diabetic).
We used both models of penile implants, CX700® and Titan®. The assignment of devices was determined by the operating surgeon through discussions with each patient. All implants were inserted through a peno-scrotal incision.
After the completion of the surgical procedure, patients were sent home on oral antibiotics for two weeks post-operatively (either cephalexin 500 mg bid or double-strength sulfamethoxazole-trimethoprim depending upon the antibiotic allergy profile of the patient).
Data were collected prospectively from each patient, including demographic information, medical history, surgical details, and follow-up information. The follow-up period was up to one year. The primary endpoint of this trial was the incidence of PP infections that necessitated implant removal. Additionally, data related to additional monitoring for allergic reactions and other serious adverse events related to exposure to CHG and the antibiotic irrigants, as well as simplicity of use and cost-effectiveness were collected.
Statistical analysis
Statistical analysis was conducted to assess the non-inferiority of 0.05% CHG compared to conventional antibiotic irrigation in preventing postoperative infections with a predetermined non inferiority threshold of 4%. Since the primary endpoint question was whether there was an infection or not, resulting in a Yes/No response, we planned to use a Chi-squared test.
Results
One hundred patients have been enrolled in our study so far (Figure 1): 50 in the 0.05% CHG arm and 50 in the conventional antibiotic arm. The recruitment process began in May 2022, with the follow-up period for our cohort extending up to 1 year as outlined in the study protocol. No patient was lost to follow up, with the last patient in this cohort completing their follow-up in January 2024. There were no statistically significant differences regarding patients’ age nor identifiable risk factors for infection i.e., diabetes mellitus, smoking status, high body mass index (BMI), immunosuppression or urinary tract instrumentation such as intermittent catheterization. As well, there were no significant difference in the number of patients who had PD or additional simple procedures i.e., circumcision, vasectomy or manual modelling for correction of residual curvature in case of PD. There were no difference in the different models of IPP used (Tables 1,2).
Table 1. Demographic criteria of 100 patients enrolled in our study.
| Demographics | CHG, N=50 | Conventional, N=50 |
|---|---|---|
| Mean age (years) | 63.94 | 62.78 |
| Risk factor of infection | ||
| Diabetes | 9 | 14 |
| High BMI | 19 | 18 |
| Smoking | 10 | 12 |
| Self-catheterizing | 1 | 0 |
| Immunosuppression | 1 | 0 |
| Peyronie’s disease | 10 | 9 |
CHG, chlorhexidine gluconate; BMI, body mass index.
Table 2. Preliminary results of 100 patients enrolled in our study.
| Surgical variables | CHG, N=50 | Conventional, N=50 |
|---|---|---|
| IPP model | ||
| CX700 | 27 | 29 |
| Titan | 22 | 21 |
| Ambicor | 1 | 0 |
| Additional procedures | 15 | 18 |
| IPP infection | 1 | 1 |
CHG, chlorhexidine gluconate; IPP, inflatable penile prosthesis.
There were two IPP infections that underwent implant removal with no salvage. One case in the CHG group developed the infection 105 days after Titan IPP placement with no known risk factor for infection; culture showed Staphylococcus aureus spp. resistant to penicillin. The other case, in the conventional antibiotic group, required cystoscopic catheter placement during the CX 700 IPP placement due to an unexpected urethral stricture found upon trial of catheter insertion. This patient subsequently developed a postoperative infection 34 days after the procedure, with a culture revealing a mix of pan-sensitive E. coli and Enterococcus spp.
No other complications related to use of either irrigation solutions i.e., allergic reaction, were reported.
Regarding cost, in our series, we used one bottle of CHG per case which offered a cost-efficient alternative (around ≈$70), especially when compared to the cumulative expenses associated with traditional approaches involving Rifampicin and Gentamicin antibiotics (≈$140 plus extra cost for pharmacy preparation). Additionally, CHG arrives ready for application in a sterile package with an extended neck bottle allowing irrigation into corporal spaces.
Discussion
Infection of PP is a devastating event for patients and surgeons as well as representing a significant financial burden on the health care system. Surgical and manufacturer efforts managed to reduce the risk of PP infection by about 50%. Current guidelines do not address the issue of which intraoperative irrigation to use during PP placement. For long, it has been a common practice to use antibiotic solutions i.e., rifampin and gentamicin for irrigation despite limited evidence to support its use in PP placement procedures.
Intra-operative irrigation during PP implantation surgery has traditionally involved the use of a combination of antibiotics such as Rifampin and Gentamicin, covering most Gram positive and negative bacteria (5). This approach aims to reduce the bacterial load within the surgical field, and consequently, to minimize the risk of infection associated with the implantation of a foreign body (the PP) into the patient’s body. While this practice has been effective in many cases, it is not without its limitations. Not only it does not cover the 3rd most common agent causing IPP infection i.e., fungal elements such as Candida spp. (7,8), one of the significant drawbacks of conventional antibiotic irrigation is the potential for the development of antibiotic resistance. Prolonged and widespread use of antibiotics can lead to the emergence of antibiotic-resistant strains of bacteria, making future infections more challenging to treat. Additionally, the process of preparing the antibiotic irrigation solutions as well as the cost, are relevant factors to be considered (10).
CHG 0.05% was approved by the Food and Drug Administration (FDA) in 2012 for utilization in irrigating and cleaning various wounds. More recently, CHG has been reintroduced in the setting of surgical wound irrigation prior to closure with biomedical implants (14,15).
Irrisept® (Irrimax Corp, Lawrenceville, Georgia, USA), 0.05% CHG concentration in sterile water, is a cationic bisbiguanide salt prepared in sterile water. The attraction of the positively charged CHG molecule to negatively charged microbial wall (bacteria, fungi and enveloped viruses) causes its disruption with subsequent cellular death (16,17). Quantitative microbial reduction counting shows that 0.05% CHG is effective against the most commonly known modern-day organisms causing penile implant infections (18). Moreover, the efficacy of CHG is not diminished in the presence of organic matter (serum) unlike the case with povidone iodine (18).
The rationale for choosing a non-inferiority study design includes ethical considerations, as using a placebo is unethical when a standard therapy exists, relevance, since non-inferiority trials are useful when a new treatment is expected to have similar efficacy but offers other advantages like cost-effectiveness or ease of use, and sample size efficiency, as non-inferiority trials typically require smaller sample sizes compared to superiority trials (19).
Defining an appropriate non-inferiority margin varies and relies on statistical reasoning and clinical judgment. In our study, non-inferiority margin was set at a 4% infection rate that fulfills a better outcome compared to historical infection rates whilst allowing marginally acceptable worse outcome compared to conventional treatment (19,20).
CHG offers several potential advantages over conventional antibiotics. First and foremost, it eliminates the risk of antibiotic resistance since it is not an antibiotic itself. This is a crucial consideration in the era of increasing antibiotic resistance, where preserving the efficacy of antibiotics is of paramount importance which is the essence of antibiotic stewardship. Moreover, CHG showcases a notable efficacy in countering fungal elements, making it a versatile solution in infection control. Fungal infection was reported as the third most common type of IPP infection (11%) as per Gross et al. (7,8).
In addition to its broad antimicrobial coverage, CHG offers the advantage of ease of administration. The simplicity of use makes it a more practical choice unlike more complex solutions that require meticulous preparation.
Furthermore, CHG stands out as a cost-effective option. Healthcare institutions and providers are constantly seeking ways to optimize resource utilization without compromising patient safety. The use of antibiotic irrigation increases time, effort, and cost due to the need for pharmacy preparation, which can cause delays and result in additional operating room time. A recently published cost effective analysis reported significant cost savings attributed to the use of CHG antiseptic as compared to antibiotic irrigation (21).
To our knowledge, this is the first prospective randomized trial addressing this specific topic of PP intraoperative irrigation solution potentially advancing the standard of care for PP implantation procedures by offering a simpler, more cost-effective, and less resistance-prone approach to intra-operative irrigation. Our research work acknowledges the evolving landscape of infection control and the importance of exploring innovative solutions that not only improve patient outcomes but also address broader healthcare challenges, such as antibiotic resistance and cost effectiveness. Through the analysis of clinical outcomes, preliminary results of this study provide insights into the potential benefits of adopting CHG as a viable alternative during de-novo PP implantation. Our study is still ongoing, recruiting more patients, expanding from two-center into multicenter as well as adopting both peno-scrotal and infra-pubic approaches.
Conclusions
Preliminary data from this non-inferiority study demonstrate that 0.05% CHG irrigation is non-inferior to conventional combined antibiotic irrigation in preventing postoperative infection following PP implantation. Moreover, the use of 0.05% CHG offers the potential advantages of ease of administration, simplifying the surgical procedure, and theoretically reducing the risk of antibiotic resistance. These findings support the consideration of 0.05% CHG as a viable alternative during PP implantation, offering both clinical effectiveness and potential cost savings. Further enrollment and analyses are ongoing to confirm these promising preliminary results and provide more robust evidence for clinical practice.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by Institutional Review Board of Rush University Medical Center (Approval No. 21120903-IRB01). The other institution (Uropartners Solaris Health) was informed and agreed with the study. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was taken from all participants.
Footnotes
Reporting Checklist: The authors have completed the CONSORT reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-24-278/rc
Trial Protocol: Available at https://tau.amegroups.com/article/view/10.21037/tau-24-278/tp
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-24-278/coif). L.A.L. received consulting fee from Irrimax Corp, Lawrenceville, GA, USA, and is an attending at Uropartners Solaris Health, Chicago, IL, USA. The other authors have no conflicts of interest to declare.
Data Sharing Statement
Available at https://tau.amegroups.com/article/view/10.21037/tau-24-278/dss
References
- 1.Swanton AR, Gross MS, Munarriz RM, et al. Penile prosthesis salvage: a historical look at the Mulcahy technique and a review of the latest literature. Int J Impot Res 2023;35:90-4. 10.1038/s41443-021-00515-7 [DOI] [PubMed] [Google Scholar]
- 2.Holland B, Kohler T. Minimizing Penile Implant Infection: A Literature Review of Patient and Surgical Factors. Curr Urol Rep 2015;16:81. 10.1007/s11934-015-0554-2 [DOI] [PubMed] [Google Scholar]
- 3.Mulcahy JJ, Köhler TS, Wen L, et al. Penile implant infection prevention part II: device coatings have changed the game. Int J Impot Res 2020;33:801-7. 10.1038/s41443-020-0338-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leong JY, Capella CE, D'Amico MJ, et al. A scoping review of penile implant biofilms-what do we know and what remains unknown? Transl Androl Urol 2022;11:1210-21. 10.21037/tau-22-195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Best JC, Clavijo RI. Best practices for infection prevention in penile prosthesis surgery. Curr Opin Urol 2020;30:302-8. 10.1097/MOU.0000000000000744 [DOI] [PubMed] [Google Scholar]
- 6.Carvajal A, Benavides J, García-Perdomo HA, et al. Risk factors associated with penile prosthesis infection: systematic review and meta-analysis. Int J Impot Res 2020;32:587-97. 10.1038/s41443-020-0232-x [DOI] [PubMed] [Google Scholar]
- 7.Gross MS, Phillips EA, Carrasquillo RJ, et al. Multicenter Investigation of the Micro-Organisms Involved in Penile Prosthesis Infection: An Analysis of the Efficacy of the AUA and EAU Guidelines for Penile Prosthesis Prophylaxis. J Sex Med 2017;14:455-63. 10.1016/j.jsxm.2017.01.007 [DOI] [PubMed] [Google Scholar]
- 8.Gross MS, Reinstatler L, Henry GD, et al. Multicenter Investigation of Fungal Infections of Inflatable Penile Prostheses. J Sex Med 2019;16:1100-5. 10.1016/j.jsxm.2019.05.003 [DOI] [PubMed] [Google Scholar]
- 9.Burnett AL, Nehra A, Breau RH, et al. Erectile Dysfunction: AUA Guideline. J Urol 2018;200:633-41. 10.1016/j.juro.2018.05.004 [DOI] [PubMed] [Google Scholar]
- 10.Blom A, Cho J, Fleischman A, et al. General Assembly, Prevention, Antiseptic Irrigation Solution: Proceedings of International Consensus on Orthopedic Infections. J Arthroplasty 2019;34:S131-8. 10.1016/j.arth.2018.09.063 [DOI] [PubMed] [Google Scholar]
- 11.Driesman A, Shen M, Feng JE, et al. Perioperative Chlorhexidine Gluconate Wash During Joint Arthroplasty Has Equivalent Periprosthetic Joint Infection Rates in Comparison to Betadine Wash. J Arthroplasty 2020;35:845-8. 10.1016/j.arth.2019.10.009 [DOI] [PubMed] [Google Scholar]
- 12.Razdan S, Siegal AR, Celtik KE, et al. Three piece penile prosthesis salvage with chlorhexidine gluconate and length preservation: our technique and outcomes. Am J Clin Exp Urol 2023;11:155-9. [PMC free article] [PubMed] [Google Scholar]
- 13.Karpman E, Griggs R, Twomey C, et al. Dipping Titan implants in Irrisept solution (0.05% chlorhexidine gluconate) and exposure to various aerobic, anaerobic, and fungal species. J Sex Med 2023;20:1025-31. 10.1093/jsxmed/qdad055 [DOI] [PubMed] [Google Scholar]
- 14.George J, Klika AK, Higuera CA. Use of Chlorhexidine Preparations in Total Joint Arthroplasty. J Bone Jt Infect 2017;2:15-22. 10.7150/jbji.16934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nguyen L, Afshari A, Green J, et al. Post-Mastectomy Surgical Pocket Irrigation With Triple Antibiotic Solution vs Chlorhexidine Gluconate: A Randomized Controlled Trial Assessing Surgical Site Infections in Immediate Tissue Expander Breast Reconstruction. Aesthet Surg J 2021;41:NP1521-8. 10.1093/asj/sjab290 [DOI] [PubMed] [Google Scholar]
- 16.Pan S, Rodriguez D, Thirumavalavan N, et al. The Use of Antiseptic Solutions in the Prevention and Management of Penile Prosthesis Infections: A Review of the Cytotoxic and Microbiological Effects of Common Irrigation Solutions. J Sex Med 2019;16:781-90. 10.1016/j.jsxm.2019.03.271 [DOI] [PubMed] [Google Scholar]
- 17.O'Donnell JA, Wu M, Cochrane NH, et al. Efficacy of common antiseptic solutions against clinically relevant microorganisms in biofilm. Bone Joint J 2021;103-B:908-15. 10.1302/0301-620X.103B5.BJJ-2020-1245.R2 [DOI] [PubMed] [Google Scholar]
- 18.Lung BE, Le R, Callan K, et al. Chlorhexidine gluconate lavage during total joint arthroplasty may improve wound healing compared to dilute betadine. J Exp Orthop 2022;9:67. 10.1186/s40634-022-00503-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mulla SM, Scott IA, Jackevicius CA, et al. How to use a noninferiority trial: users' guides to the medical literature. JAMA 2012;308:2605-11. 10.1001/2012.jama.11235 [DOI] [PubMed] [Google Scholar]
- 20.U.S. Food and Drug Administration. Non-Inferiority Clinical Trials to Establish Effectiveness Guidance for Industry (November 2016). Available online: www.fda.gov/regulatory-information/search-fda-guidance-documents/non-inferiority-clinical-trials
- 21.McDermott S, Kaiser M, Deibert C. Use of Chlorhexidine Gluconate Irrigation Solution to Prevent Postoperative Infection in Inflatable Penile Prosthesis: a Cost-effectiveness Analysis. Research Square; 2022. DOI: . 10.21203/rs.3.rs-1755090/v1 [DOI]