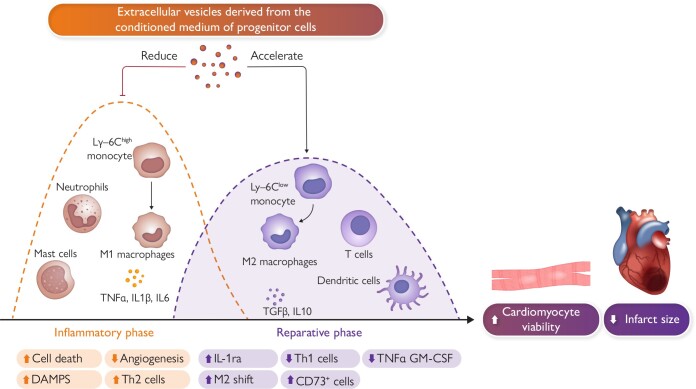
Graphical Abstract.
Inflammation plays a significant role in acute myocardial infarction, affecting both the early phase following the clinical presentation of acute coronary syndrome and the subsequent chronic phase. During the acute phase, various inflammatory cell types, such as T-cells, granulocytes, monocytes, and B-cells, are attracted to the infarcted area to remove necrotic tissue and promote scar tissue formation. Initially, pro-inflammatory cells like polymorphonuclear neutrophils and M1 pro-inflammatory macrophages are predominant. Within 24–48 h, these M1 macrophages transition into an anti-inflammatory M2 state, contributing to the healing process. T-cells, including CD8+ and CD4+ types, are activated within lymph nodes connected to the heart and play a role in the healing process. Experiments conducted in small and large animals suggest a potential therapeutic role for exogenous extracellular vesicles (EVs) as an adjunctive therapy to percutaneous coronary intervention. EV-mediated modulation of the immune response facilitates a transition from an inflammatory state to a resolving phase after ischaemic damage. Abbreviations: DAMPS, damage-associated molecular pattern molecules; GM-CSF, granulocyte-macrophage colony stimulating factor; IL, Interleukin; IL-1ra, interleukin-1 receptor antagonist; Ly-6C, lymphocyte antigen; M1, inflammatory monocytes/macrophages; M2, reparative monocytes/macrophages; TGFB, transforming growth factor beta; Th2, T-helper cells; TNF-α, tumour necrosis factor alpha.