Abstract
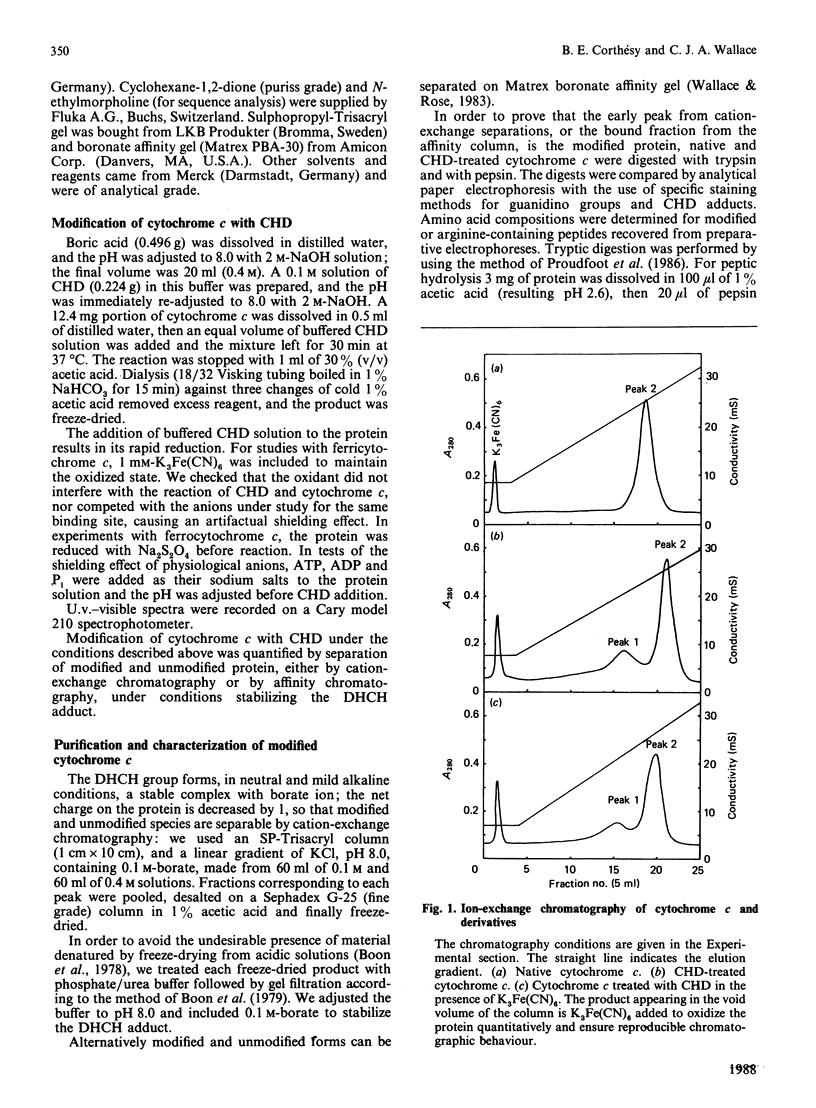
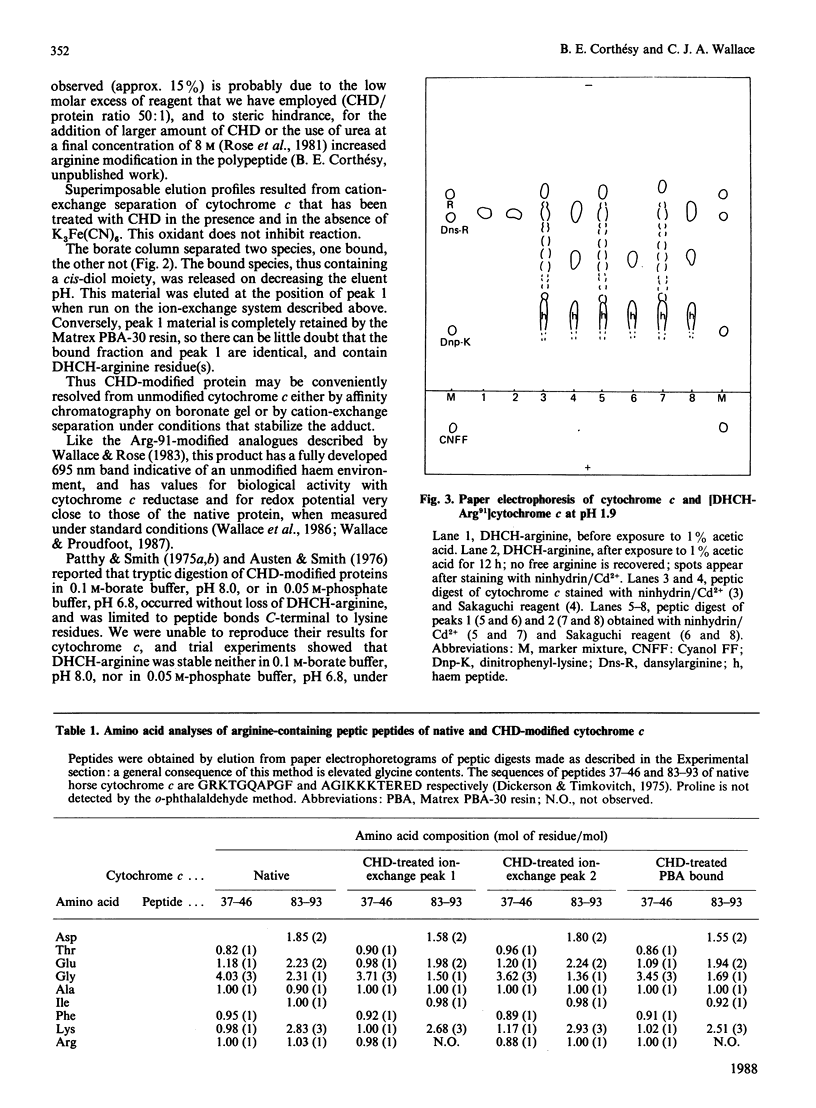
Arg-91 is not part of the active site of cytochrome c that mediates binding and electron transfer, yet it is absolutely conserved in eukaryotic cytochromes c, indicating a special function. The physicochemical properties of analogues are unaffected by the modification of this residue, so they can be used with confidence to study the role of Arg-91. We have established limiting conditions under which this residue alone is specifically modified by cyclohexane-1,2-dione, and have subsequently shown that ATP, and to a lesser extent ADP or Pi, protects it from the action of the reagent in an oxidation-state-dependent manner. These observations strongly support the idea that this site exerts a controlling influence on cytochrome c activity in the electron transport or other cellular redox systems, and we have commenced a study of how that influence might operate. We find that the redox potentials of both cytochrome c and analogue are little affected by changing ATP or Pi concentrations.
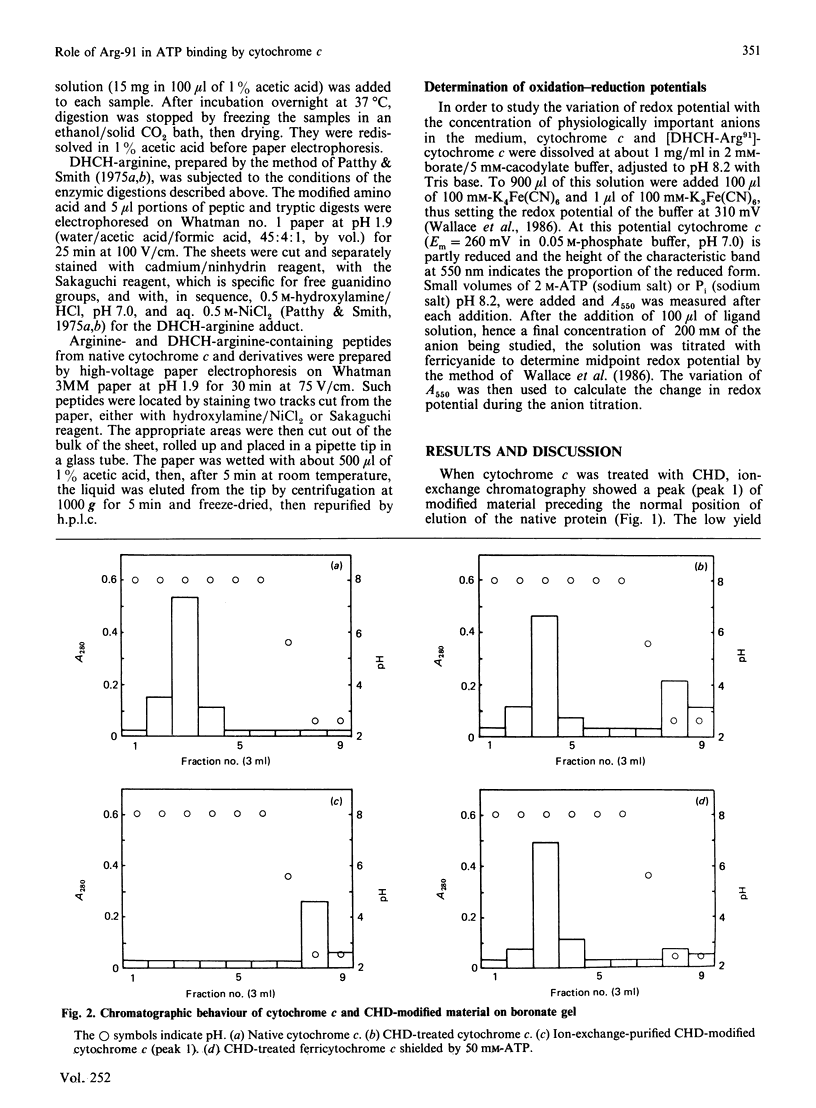
Full text
PDF
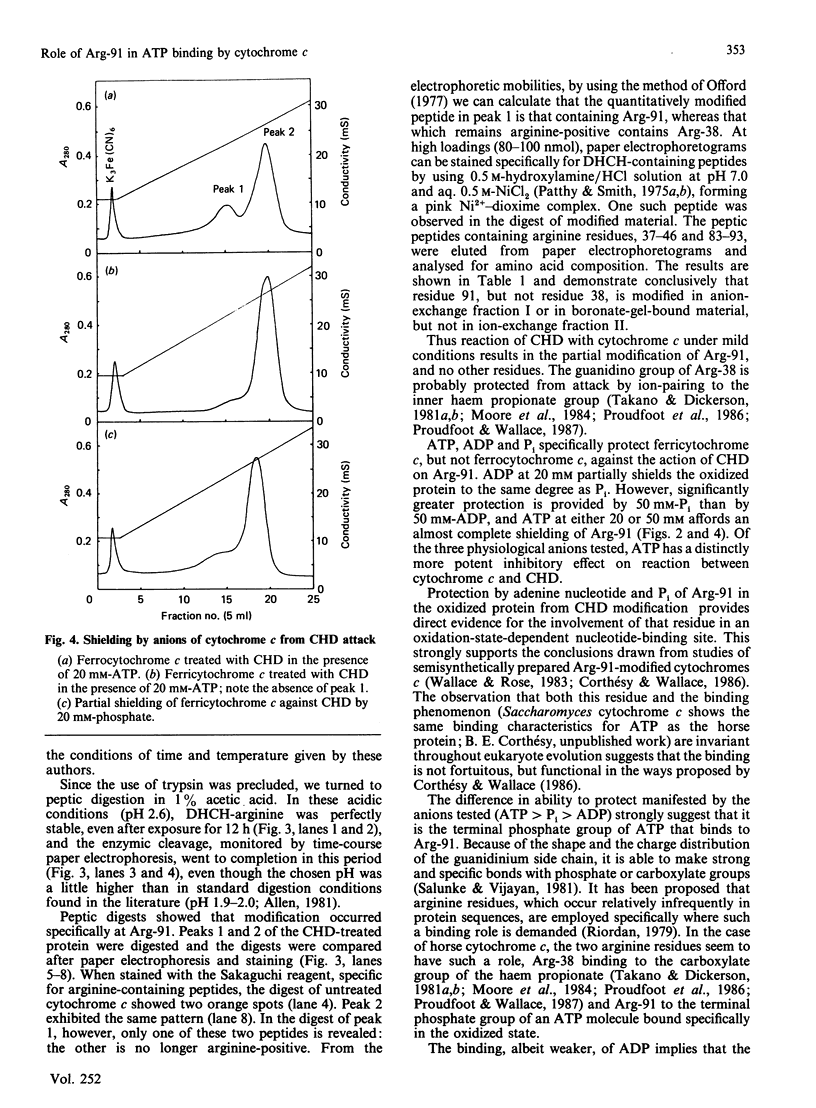

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austen B. M., Smith E. L. Identification of a functional arginine residue involved in coenzyme binding by the NADP-specific glutamate dehydrogenase of Neurospora. J Biol Chem. 1976 Sep 25;251(18):5835–5837. [PubMed] [Google Scholar]
- Bill K., Azzi A. Interaction of reduced and oxidized cytochrome c with the mitochondrial cytochrome c oxidase and bc1-complex. Biochem Biophys Res Commun. 1984 Apr 16;120(1):124–130. doi: 10.1016/0006-291x(84)91422-0. [DOI] [PubMed] [Google Scholar]
- Boon P. J., Tesser G. I., Nivard R. J. Semisynthetic horse heart [65-homoserine]cytochrome c from three fragments. Proc Natl Acad Sci U S A. 1979 Jan;76(1):61–65. doi: 10.1073/pnas.76.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corthésy B. E., Wallace C. J. The oxidation-state-dependent ATP-binding site of cytochrome c. A possible physiological significance. Biochem J. 1986 Jun 1;236(2):359–364. doi: 10.1042/bj2360359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson R. E. The structure and history of an ancient protein. Sci Am. 1972 Apr;226(4):58–passim. doi: 10.1038/scientificamerican0472-58. [DOI] [PubMed] [Google Scholar]
- Ferguson-Miller S., Brautigan D. L., Margoliash E. Correlation of the kinetics of electron transfer activity of various eukaryotic cytochromes c with binding to mitochondrial cytochrome c oxidase. J Biol Chem. 1976 Feb 25;251(4):1104–1115. [PubMed] [Google Scholar]
- Ferguson-Miller S., Brautigan D. L., Margoliash E. Definition of cytochrome c binding domains by chemical modification. III. Kinetics of reaction of carboxydinitrophenyl cytochromes c with cytochrome c oxidase. J Biol Chem. 1978 Jan 10;253(1):149–159. [PubMed] [Google Scholar]
- Kadenbach B. Regulation of respiration and ATP synthesis in higher organisms: hypothesis. J Bioenerg Biomembr. 1986 Feb;18(1):39–54. doi: 10.1007/BF00743611. [DOI] [PubMed] [Google Scholar]
- Margoliash E., Schejter A. Cytochrome c. Adv Protein Chem. 1966;21:113–286. doi: 10.1016/s0065-3233(08)60128-x. [DOI] [PubMed] [Google Scholar]
- Offord R. E. The use of logarithmic plots of electrophoretic mobilities of peptides. Methods Enzymol. 1977;47:51–69. doi: 10.1016/0076-6879(77)47008-3. [DOI] [PubMed] [Google Scholar]
- Patthy L., Smith E. L. Identification of functional arginine residues in ribonuclease A and lysozyme. J Biol Chem. 1975 Jan 25;250(2):565–569. [PubMed] [Google Scholar]
- Patthy L., Smith E. L. Reversible modification of arginine residues. Application to sequence studies by restriction of tryptic hydrolysis to lysine residues. J Biol Chem. 1975 Jan 25;250(2):557–564. [PubMed] [Google Scholar]
- Petersen L. C. Cytochrome c--cytochrome aa3 complex formation at low ionic strength studied by aqueous two-phase partition. FEBS Lett. 1978 Oct 1;94(1):105–108. doi: 10.1016/0014-5793(78)80916-8. [DOI] [PubMed] [Google Scholar]
- Proudfoot A. E., Wallace C. J., Harris D. E., Offord R. E. A new non-covalent complex of semisynthetically modified tryptic fragments of cytochrome c. Biochem J. 1986 Oct 15;239(2):333–337. doi: 10.1042/bj2390333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot A. E., Wallace C. J. Semisynthesis of cytochrome c analogues. The effect of modifying the conserved residues 38 and 39. Biochem J. 1987 Dec 15;248(3):965–967. doi: 10.1042/bj2480965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees D. C. Experimental evaluation of the effective dielectric constant of proteins. J Mol Biol. 1980 Aug 15;141(3):323–326. doi: 10.1016/0022-2836(80)90184-9. [DOI] [PubMed] [Google Scholar]
- Riordan J. F. Arginyl residues and anion binding sites in proteins. Mol Cell Biochem. 1979 Jul 31;26(2):71–92. doi: 10.1007/BF00232886. [DOI] [PubMed] [Google Scholar]
- Salunke D. M., Vijayan M. Specific interactions involving guanidyl group observed in crystal structures. Int J Pept Protein Res. 1981 Oct;18(4):348–351. doi: 10.1111/j.1399-3011.1981.tb02992.x. [DOI] [PubMed] [Google Scholar]
- Takano T., Dickerson R. E. Conformation change of cytochrome c. I. Ferrocytochrome c structure refined at 1.5 A resolution. J Mol Biol. 1981 Nov 25;153(1):79–94. doi: 10.1016/0022-2836(81)90528-3. [DOI] [PubMed] [Google Scholar]
- Takano T., Dickerson R. E. Conformation change of cytochrome c. II. Ferricytochrome c refinement at 1.8 A and comparison with the ferrocytochrome structure. J Mol Biol. 1981 Nov 25;153(1):95–115. doi: 10.1016/0022-2836(81)90529-5. [DOI] [PubMed] [Google Scholar]
- Wallace C. J., Corradin G., Marchiori F., Borin G. Cytochrome c chimerae from natural and synthetic fragments: significance of the biological properties. Biopolymers. 1986 Nov;25(11):2121–2132. doi: 10.1002/bip.360251107. [DOI] [PubMed] [Google Scholar]
- Wallace C. J., Corthésy B. E. Protein engineering of cytochrome c by semisynthesis: substitutions at glutamic acid 66. Protein Eng. 1986 Oct-Nov;1(1):23–27. [PubMed] [Google Scholar]
- Wallace C. J. Functional consequences of the excision of an omega loop, residues 40-55, from mitochondrial cytochrome c. J Biol Chem. 1987 Dec 15;262(35):16767–16770. [PubMed] [Google Scholar]
- Wallace C. J., Proudfoot A. E. On the relationship between oxidation-reduction potential and biological activity in cytochrome c analogues. Results from four novel two-fragment complexes. Biochem J. 1987 Aug 1;245(3):773–779. doi: 10.1042/bj2450773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace C. J., Rose K. The semisynthesis of analogues of cytochrome c. Modifications of arginine residues 38 and 91. Biochem J. 1983 Dec 1;215(3):651–658. doi: 10.1042/bj2150651. [DOI] [PMC free article] [PubMed] [Google Scholar]


