Abstract
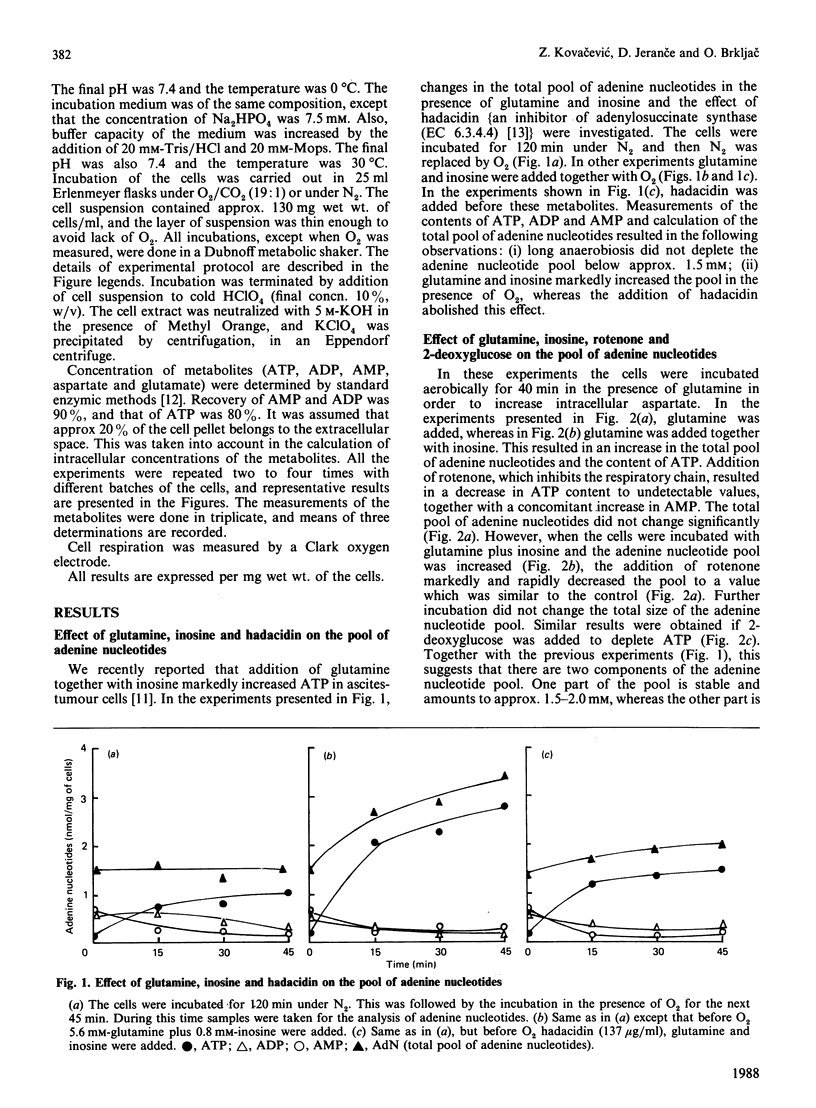
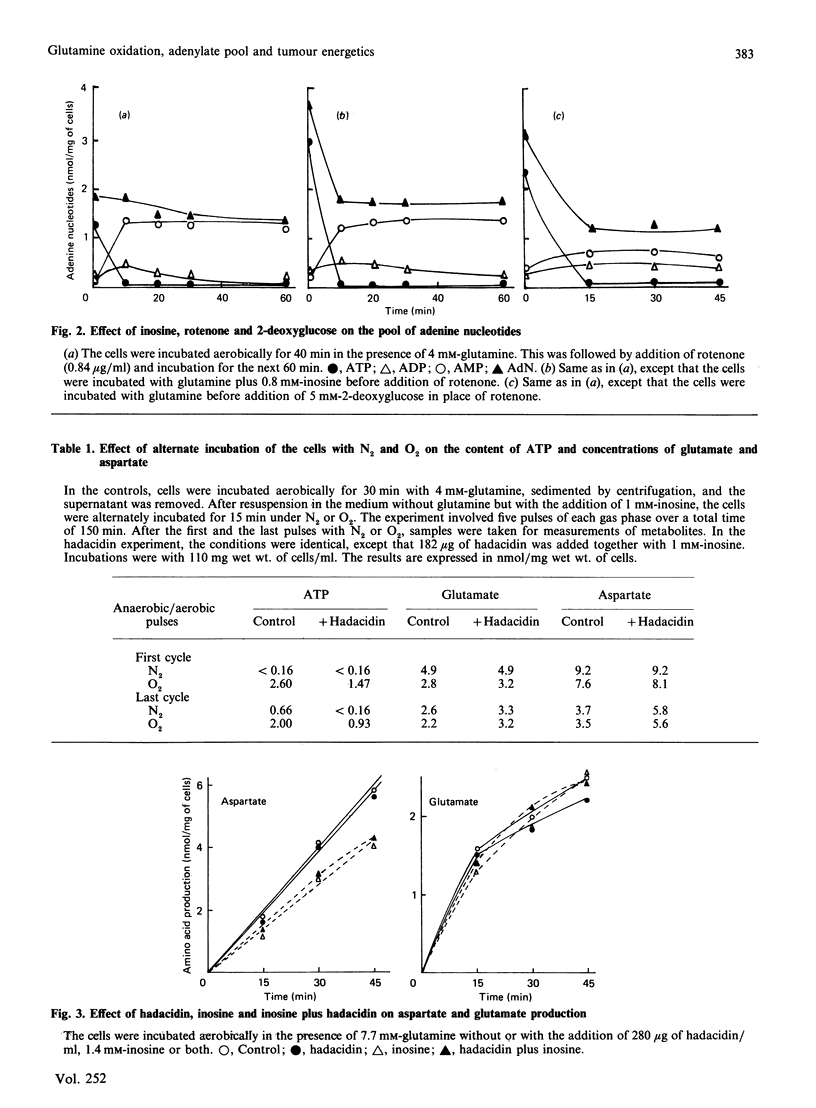
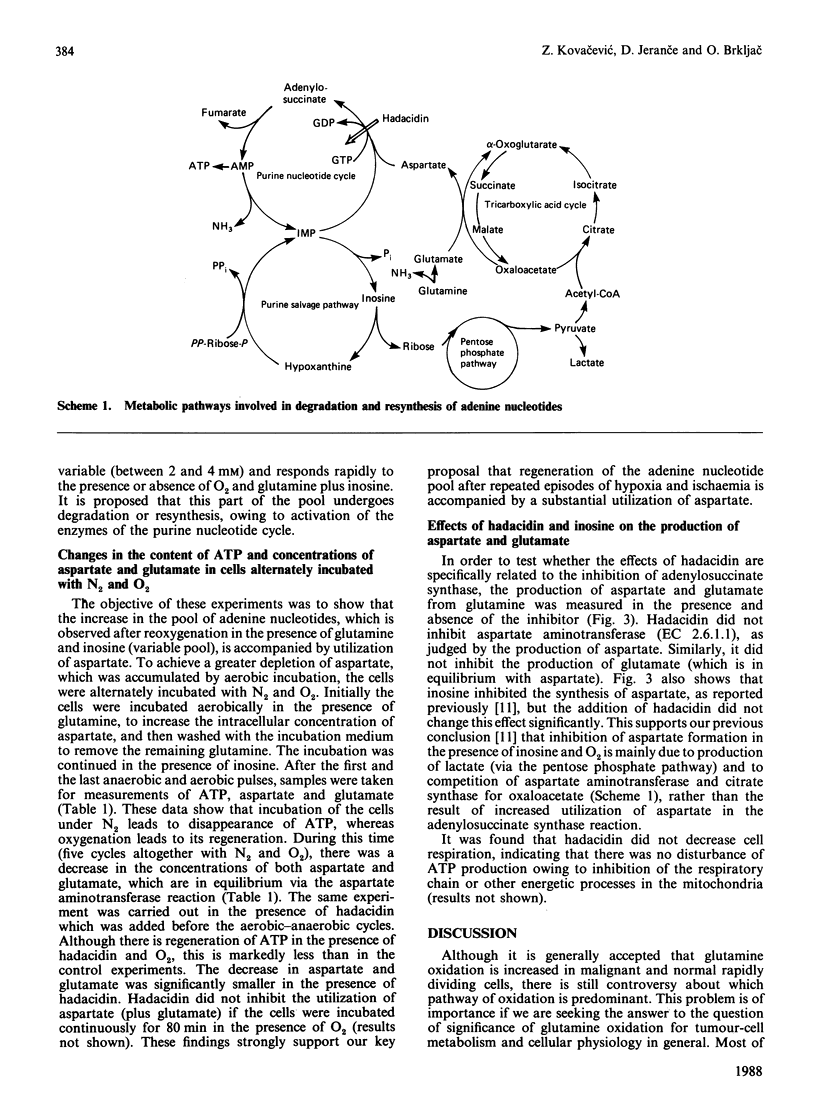
It is proposed that the purine nucleotide cycle and glutamine oxidation play a key role in the adaptation of tumour energetics to the transition from the anaerobic to the aerobic state. In support of this proposal, it was found that glutamine and inosine markedly increase total adenylates in the presence of oxygen, whereas the addition of hadacidin abolishes this effect. Transition of the cells from the anaerobic to the aerobic state, and vice versa, in the presence of glutamine plus inosine revealed that there are two components of the adenine nucleotide pool, one which is stable and the other which is variable and responds to the aerobic-anaerobic transition. This part of the pool undergoes degradation or resynthesis owing to activation of the enzymes of the purine nucleotide cycle. Resynthesis of the pool is accompanied by substantial net utilization of aspartate, which is produced by glutamine oxidation. This is supported by the experiments in which the cells were alternately incubated with nitrogen or oxygen, demonstrating that hadacidin significantly decreased utilization of aspartate and regeneration of ATP owing to inhibition of adenylosuccinate synthase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aragón J. J., Tornheim K., Goodman M. N., Lowenstein J. M. Replenishment of citric acid cycle intermediates by the purine nucleotide cycle in rat skeletal muscle. Curr Top Cell Regul. 1981;18:131–149. doi: 10.1016/b978-0-12-152818-8.50014-1. [DOI] [PubMed] [Google Scholar]
- Bates D. J., Perrett D., Mowbray J. Systematic variations in the content of the purine nucleotides in the steady-state perfused rat heart. Evidence for the existence of controlled storage and release of adenine nucleotides. Biochem J. 1978 Nov 15;176(2):485–493. doi: 10.1042/bj1760485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman A. G., Miller A. L., Atkinson D. E. Role of the adenylate deaminase reaction in regulation of adenine nucleotide metabolism in Ehrlich ascites tumor cells. Cancer Res. 1976 Mar;36(3):1144–1150. [PubMed] [Google Scholar]
- Hutchinson W. L., Morris P. G., Mowbray J. The molecular structure of a rapidly formed oligomeric adenosine tetraphosphate derivative from rat heart. Biochem J. 1986 Mar 15;234(3):623–627. doi: 10.1042/bj2340623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson W. L., Ratcliffe P. J., Mowbray J. Evidence for the presence of oligophosphoglyceroyl-ATP in rat offney. Biochem J. 1986 Dec 1;240(2):597–599. doi: 10.1042/bj2400597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelleher J. K., Bryan B. M., 3rd, Mallet R. T., Holleran A. L., Murphy A. N., Fiskum G. Analysis of tricarboxylic acid-cycle metabolism of hepatoma cells by comparison of 14CO2 ratios. Biochem J. 1987 Sep 15;246(3):633–639. doi: 10.1042/bj2460633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch W. M., Schulz Q., Van Buskirk J., Nakane P. Anaerobic energy metabolism in brain tumors. Prog Exp Tumor Res. 1972;17:163–191. doi: 10.1159/000393673. [DOI] [PubMed] [Google Scholar]
- Kovacevic Z., McGivan J. D. Mitochondrial metabolism of glutamine and glutamate and its physiological significance. Physiol Rev. 1983 Apr;63(2):547–605. doi: 10.1152/physrev.1983.63.2.547. [DOI] [PubMed] [Google Scholar]
- Kovacević Z., Morris H. P. The role of glutamine in the oxidative metabolism of malignant cells. Cancer Res. 1972 Feb;32(2):326–333. [PubMed] [Google Scholar]
- Kovacević Z., Popović J., Brkljac O., Lelas S. Interaction of metabolism of aspartate and inosine and energy state of malignant cells. Biochem J. 1987 Oct 1;247(1):47–51. doi: 10.1042/bj2470047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacević Z. Possibility for the transfer of reducing equivalents from the cytosol to the mitochondrial compartment in Ehrlich ascites tumor cells by the malate-aspartate shuttle. Eur J Biochem. 1972 Feb 15;25(2):372–378. doi: 10.1111/j.1432-1033.1972.tb01706.x. [DOI] [PubMed] [Google Scholar]
- Moreadith R. W., Lehninger A. L. The pathways of glutamate and glutamine oxidation by tumor cell mitochondria. Role of mitochondrial NAD(P)+-dependent malic enzyme. J Biol Chem. 1984 May 25;259(10):6215–6221. [PubMed] [Google Scholar]
- Newsholme E. A., Crabtree B., Ardawi M. S. The role of high rates of glycolysis and glutamine utilization in rapidly dividing cells. Biosci Rep. 1985 May;5(5):393–400. doi: 10.1007/BF01116556. [DOI] [PubMed] [Google Scholar]
- Parlo R. A., Coleman P. S. Enhanced rate of citrate export from cholesterol-rich hepatoma mitochondria. The truncated Krebs cycle and other metabolic ramifications of mitochondrial membrane cholesterol. J Biol Chem. 1984 Aug 25;259(16):9997–10003. [PubMed] [Google Scholar]
- Pedersen P. L. Tumor mitochondria and the bioenergetics of cancer cells. Prog Exp Tumor Res. 1978;22:190–274. doi: 10.1159/000401202. [DOI] [PubMed] [Google Scholar]
- Sauer L. A., Dauchy R. T., Nagel W. O., Morris H. P. Mitochondrial malic enzymes. Mitochondrial NAD(P)+-dependent malic enzyme activity and malate-dependent pyruvate formation are progression-linked in Morris hepatomas. J Biol Chem. 1980 May 10;255(9):3844–3848. [PubMed] [Google Scholar]
- Stayton M. M., Rudolph F. B., Fromm H. J. Regulation, genetics, and properties of adenylosuccinate synthetase: a review. Curr Top Cell Regul. 1983;22:103–141. doi: 10.1016/b978-0-12-152822-5.50008-7. [DOI] [PubMed] [Google Scholar]
- Weber G., Prajda N., Jackson R. C. Key enzymes of IMP metabolism: transformation and proliferation-linked alterations in gene expression. Adv Enzyme Regul. 1976;14:3–24. doi: 10.1016/0065-2571(76)90005-4. [DOI] [PubMed] [Google Scholar]


