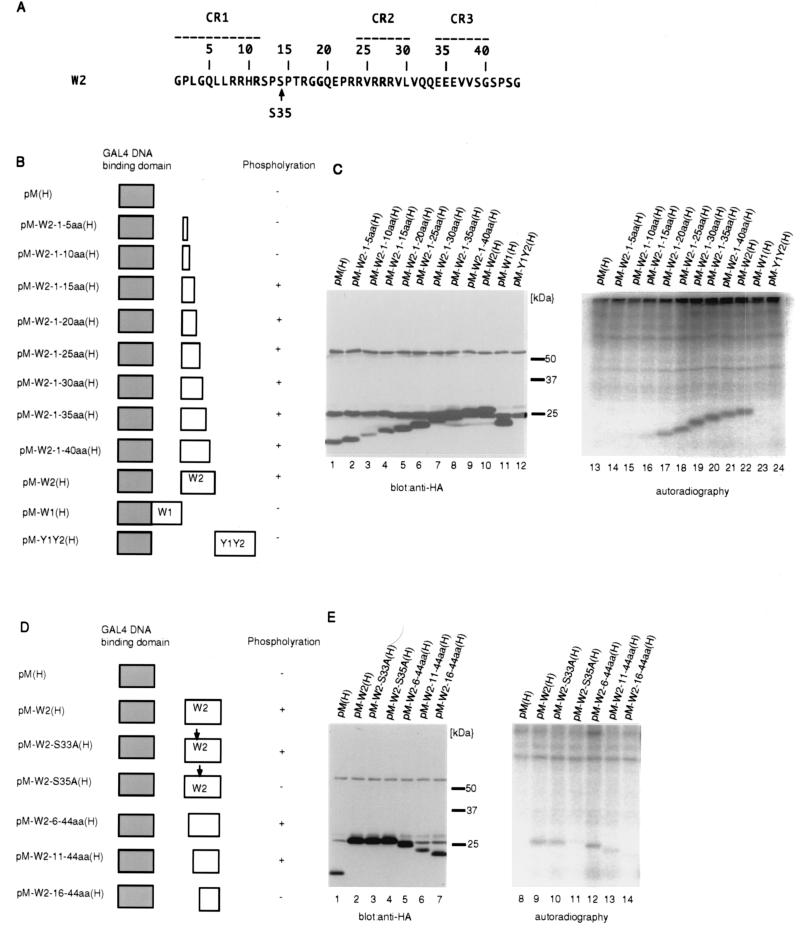
FIG. 4.
Identification of the major phosphorylation site in EBNA-LP. (A) The amino acid sequence of the W2 exon of EBNA-LP. The numbering of amino acids (relative to the first codon of the W2 domain) is shown above the sequence. Conserved regions defined by Peng et al. (27) are indicated as broken lines with their designation given above. The conserved serine that was found to be phosphorylated is indicated by an arrow. (B) Schematic representation of various deletion mutants of HA-tagged EBNA-LP domains fused to the GAL4 DNA binding domain. The levels of phosphorylation of the mutants are also shown. (C) BOSC23 cells were transiently transfected with the indicated expression vectors described in panel B, labeled with [32P]orthophosphate for 4 h, and then harvested, solubilized, immunoprecipitated with anti-GAL4 DNA binding domain monoclonal antibody (RK5C1), and electrophoretically separated in duplicate on two denaturing gels. Each gel was subjected to immunoblotting with anti-HA monoclonal antibody (3F10) (lanes 1 to 12) or autoradiography (lanes 13 to 24). (D) Schematic representation of various deletion or substitution mutants of the HA-tagged W2 domain fused to the GAL4 DNA binding domain. The levels of phosphorylation of the mutants are also shown. (E) Autoradiographic and photographic images of 32P-radiolabeled transfected BOSC23 cell lysates that were immunoprecipitated with anti-GAL4 DNA binding domain monoclonal antibody (RK5C1) and subjected to immunoblotting with anti-HA monoclonal antibody (3F10) (lanes 1 to 7) or autoradiography (lanes 8 to 14). Experiments were done exactly as described in the legend to panel C, except that BOSC23 cells were transfected with the indicated expression vectors described in panel D.