Abstract
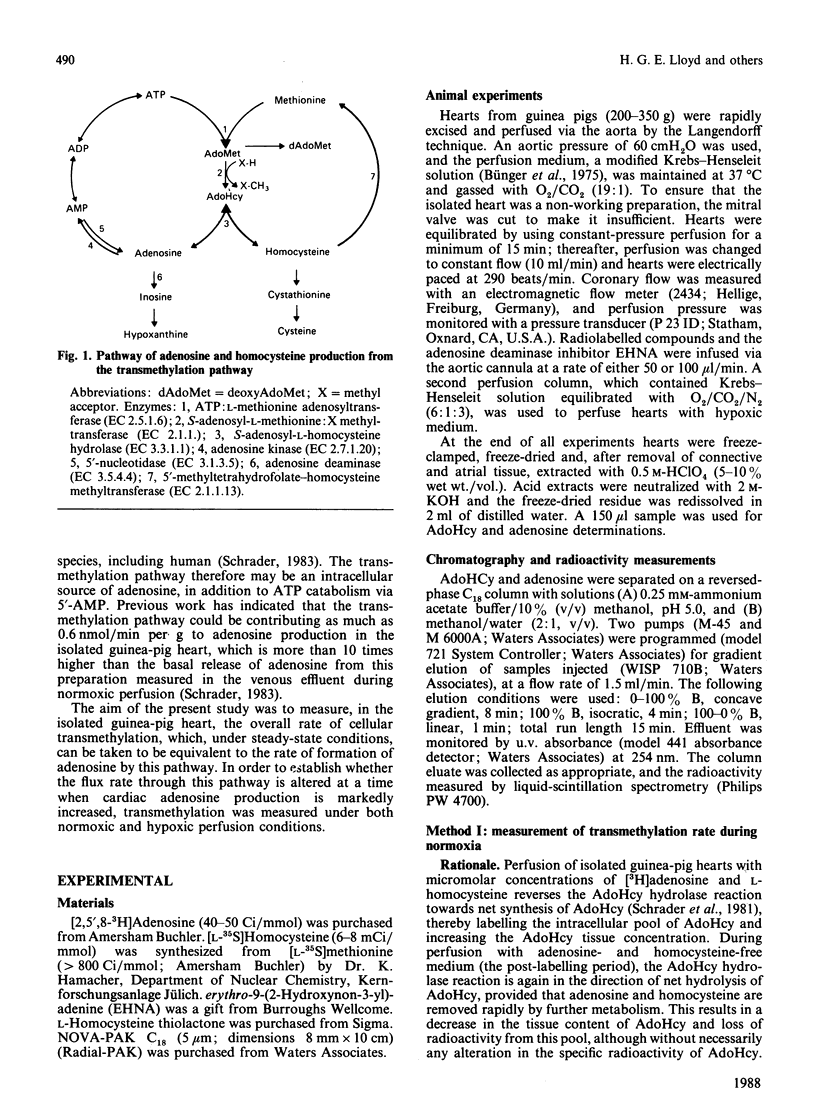
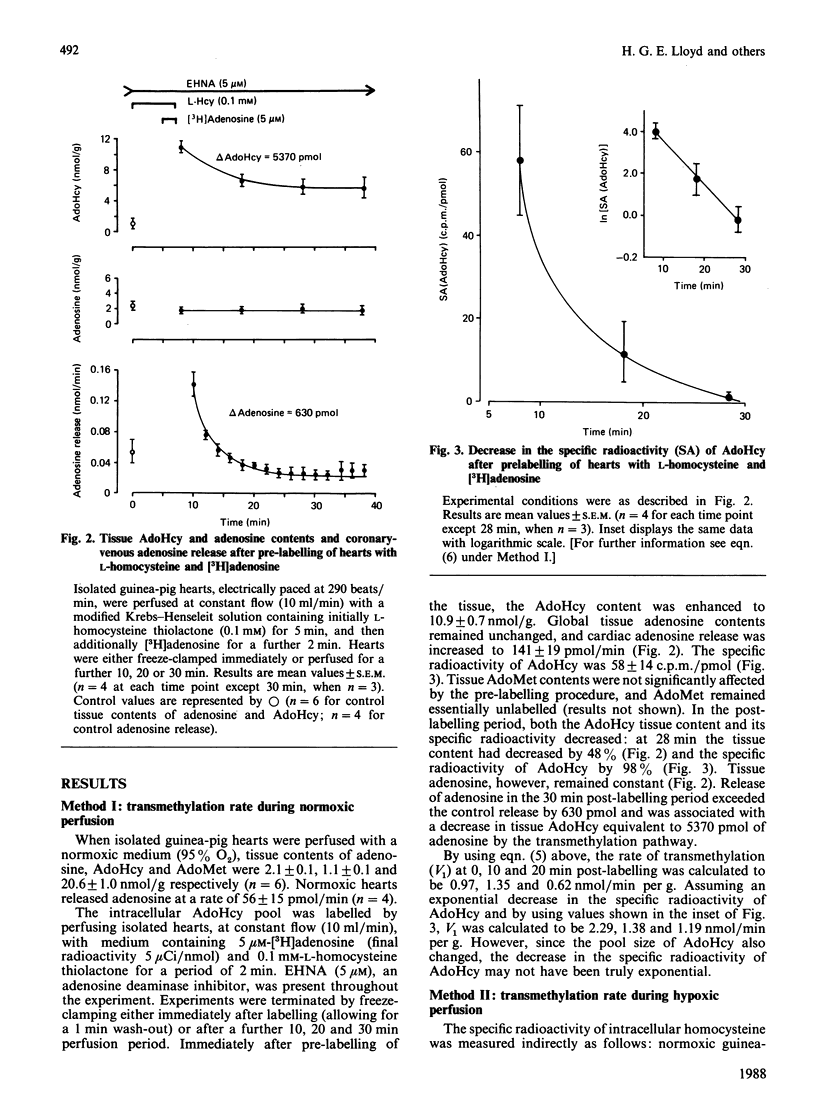
In order to quantify adenosine production from the transmethylation pathway [S-adenosylmethionine (AdoMet)----S-adenosylhomocysteine (AdoHcy) in equilibrium adenosine + L-homocysteine] in the isolated guinea-pig heart under basal conditions (normoxic perfusion with 95% O2) and during elevated adenosine production (hypoxic perfusion with 30% O2), two methods were used. (1) Hearts were perfused with normoxic medium containing [2,5,8-3H]adenosine (5 microM) and L-homocysteine thiolactone (0.1 mM), which brings about net AdoHcy synthesis via reversal of the AdoHcy hydrolase reaction and labels the intracellular pool of AdoHcy. From the decrease in AdoHcy pool size and specific radioactivity of AdoHcy in the post-labelling period, the rate of transmethylation, which is equivalent to the rate of adenosine production, was calculated to be 0.98 nmol/min per g. Adenosine release from the hearts was 40-50 pmol/min per g. (2) Hearts were perfused with hypoxic medium containing [35S]homocysteine (50 microM). Owing to the hypoxia-induced increase in adenosine production, this procedure also results in expansion and labelling of the AdoHcy pool. From the dilution of the specific radioactivity of AdoHcy relative to that of [35S]homocysteine, the rate of AdoHcy synthesis from AdoMet (transmethylation) was calculated to be 1.12 nmol/min per g. It is concluded that in the oxygenated heart the transmethylation pathway is quantitatively an important intracellular source of adenosine, which exceeds the rate of adenosine wash-out by the coronary system by about 15-fold. Most of the adenosine formed by this pathway is re-incorporated into the ATP pool, most likely by adenosine kinase. The transmethylation pathway is essentially O2-independent, and the known hypoxia-induced production of adenosine must be derived from an increase in 5'-AMP hydrolysis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERNE R. M. Cardiac nucleotides in hypoxia: possible role in regulation of coronary blood flow. Am J Physiol. 1963 Feb;204:317–322. doi: 10.1152/ajplegacy.1963.204.2.317. [DOI] [PubMed] [Google Scholar]
- Baer H. P., Drummond G. I. Catabolism of adenine nucleotides by the isolated perfused rat heart. Proc Soc Exp Biol Med. 1968 Jan;127(1):33–36. doi: 10.3181/00379727-127-32614. [DOI] [PubMed] [Google Scholar]
- Bardenheuer H., Schrader J. Supply-to-demand ratio for oxygen determines formation of adenosine by the heart. Am J Physiol. 1986 Feb;250(2 Pt 2):H173–H180. doi: 10.1152/ajpheart.1986.250.2.H173. [DOI] [PubMed] [Google Scholar]
- Berne R. M. The role of adenosine in the regulation of coronary blood flow. Circ Res. 1980 Dec;47(6):807–813. doi: 10.1161/01.res.47.6.807. [DOI] [PubMed] [Google Scholar]
- Bünger R., Haddy F. J., Gerlach E. Coronary responses to dilating substances and competitive inhibition by theophylline in the isolated perfused guinea pig heart. Pflugers Arch. 1975 Jul 28;358(3):213–224. doi: 10.1007/BF00587218. [DOI] [PubMed] [Google Scholar]
- CATONI G. L. S-Adenosylmethionine; a new intermediate formed enzymatically from L-methionine and adenosinetriphosphate. J Biol Chem. 1953 Sep;204(1):403–416. [PubMed] [Google Scholar]
- DE LA HABA G., CANTONI G. L. The enzymatic synthesis of S-adenosyl-L-homocysteine from adenosine and homocysteine. J Biol Chem. 1959 Mar;234(3):603–608. [PubMed] [Google Scholar]
- Drury A. N., Szent-Györgyi A. The physiological activity of adenine compounds with especial reference to their action upon the mammalian heart. J Physiol. 1929 Nov 25;68(3):213–237. doi: 10.1113/jphysiol.1929.sp002608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman J. L. The rate of transmethylation in mouse liver as measured by trapping S-adenosylhomocysteine. Arch Biochem Biophys. 1980 Nov;205(1):132–135. doi: 10.1016/0003-9861(80)90091-0. [DOI] [PubMed] [Google Scholar]
- Kroll K., Schrader J., Piper H. M., Henrich M. Release of adenosine and cyclic AMP from coronary endothelium in isolated guinea pig hearts: relation to coronary flow. Circ Res. 1987 May;60(5):659–665. doi: 10.1161/01.res.60.5.659. [DOI] [PubMed] [Google Scholar]
- Meghji P., Holmquist C. A., Newby A. C. Adenosine formation and release from neonatal-rat heart cells in culture. Biochem J. 1985 Aug 1;229(3):799–805. doi: 10.1042/bj2290799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nees S., Herzog V., Becker B. F., Böck M., Des Rosiers Ch, Gerlach E. The coronary endothelium: a highly active metabolic barrier for adenosine. Basic Res Cardiol. 1985 Sep-Oct;80(5):515–529. doi: 10.1007/BF01907915. [DOI] [PubMed] [Google Scholar]
- Rubio R., Berne R. M., Dobson J. G., Jr Sites of adenosine production in cardiac and skeletal muscle. Am J Physiol. 1973 Oct;225(4):938–953. doi: 10.1152/ajplegacy.1973.225.4.938. [DOI] [PubMed] [Google Scholar]
- Schrader J., Haddy F. J., Gerlach E. Release of adenosine, inosine and hypoxanthine from the isolated guinea pig heart during hypoxia, flow-autoregulation and reactive hyperemia. Pflugers Arch. 1977 May 6;369(1):1–6. doi: 10.1007/BF00580802. [DOI] [PubMed] [Google Scholar]
- Schrader J., Schütz W., Bardenheuer H. Role of S-adenosylhomocysteine hydrolase in adenosine metabolism in mammalian heart. Biochem J. 1981 Apr 15;196(1):65–70. doi: 10.1042/bj1960065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schütz W., Schrader J., Gerlach E. Different sites of adenosine formation in the heart. Am J Physiol. 1981 Jun;240(6):H963–H970. doi: 10.1152/ajpheart.1981.240.6.H963. [DOI] [PubMed] [Google Scholar]
- Watkins C. A., Morgan H. E. Relationship between rates of methylation and synthesis of heart protein. J Biol Chem. 1979 Feb 10;254(3):693–701. [PubMed] [Google Scholar]


