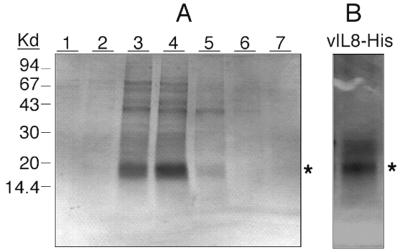
FIG. 5.
Purification of six-His-tagged vIL-8 produced by baculovirus expression system. (A) Purification of recombinant vIL-8–His by Ni-affinity column chromatography. Cell-free supernatants collected from High Five insect cells infected with vIL-8–His recombinant baculovirus were prepared and loaded onto a Ni column as described in Materials and Methods. Bound vIL-8–His was eluted by a 20 to 500 mM imidazole gradient. Twenty microliters of each elution fraction was loaded onto an SDS–10 to 20% PAGE gel, separated electrophoretically, and stained with Coomassie blue. Kd, kilodaltons. (B) Silver staining demonstrates the purity of vIL-8–His after gel filtration. Elution fractions 3 and 4 of the Ni column were pooled, concentrated eightfold by Centricon-3 spin concentration, and further purified by gel filtration chromatography (Superose 12). Fractions were collected every 2 min at a flow rate of 0.4 min/ml. Purity of the eluent with the highest chemotaxis activity (42- to 44-min fractions) was analyzed by SDS–10 to 20% PAGE followed by silver staining. A major protein band with a size of approximately 20 kDa is indicated with an asterisk.