Abstract
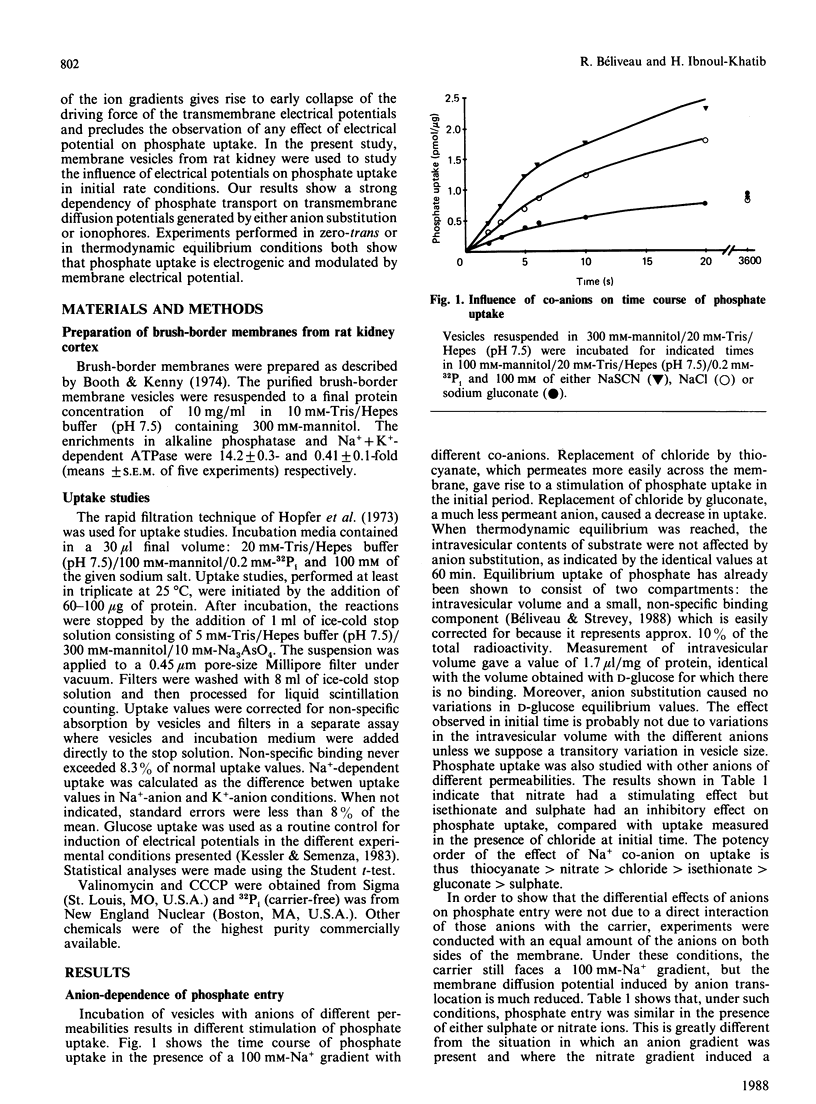
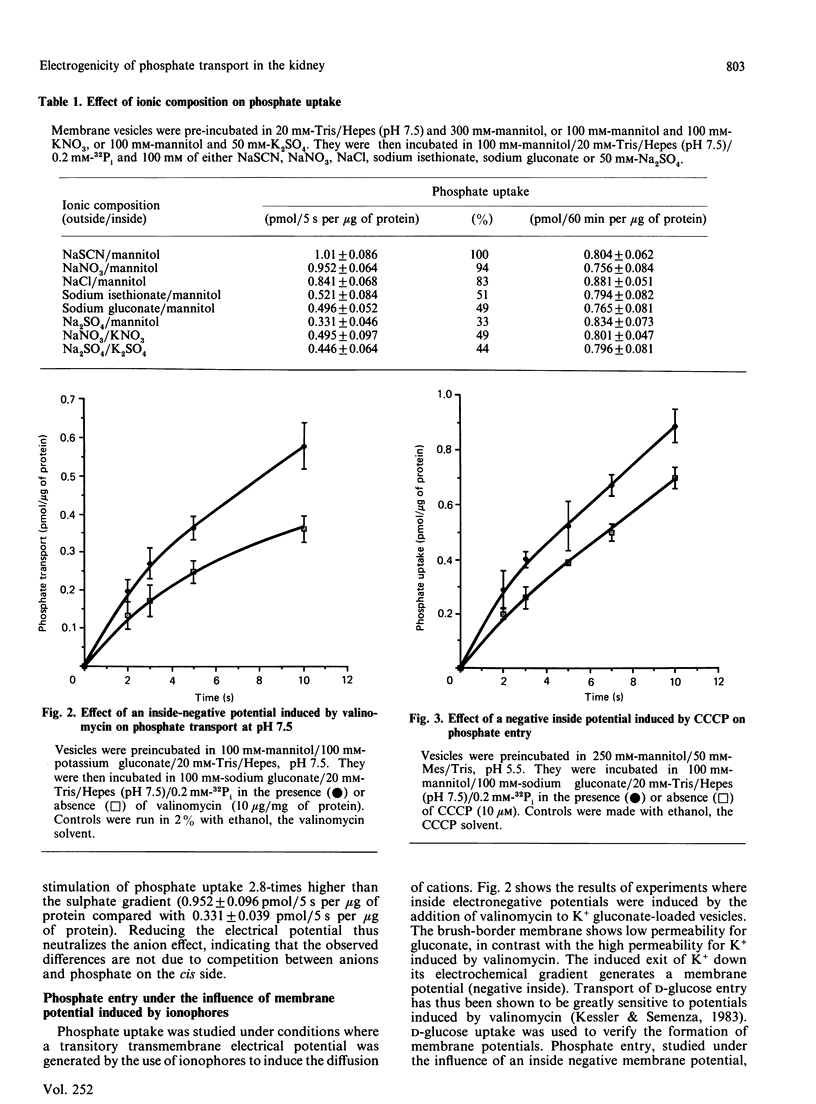
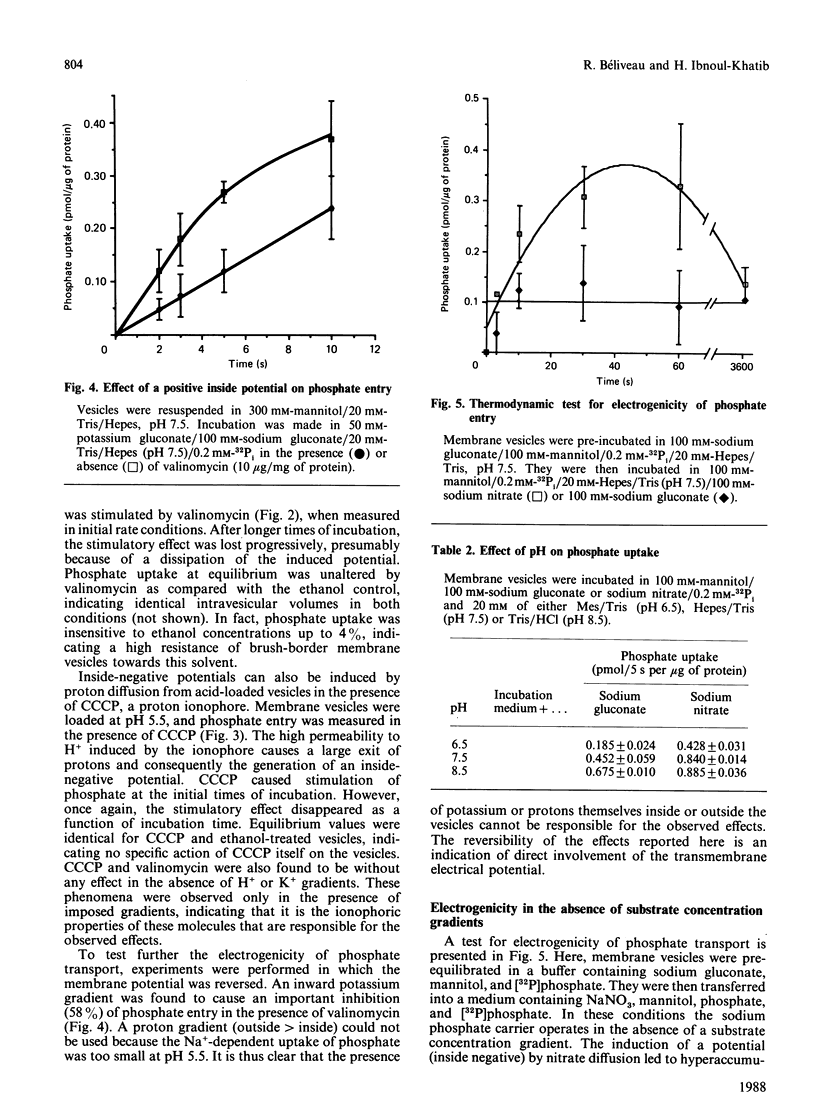
Phosphate uptake by rat renal brush-border membrane vesicles was studied under experimental conditions where transmembrane electrical potential (delta psi) could be manipulated. Experiments were performed under initial rate conditions to avoid complications associated with the dissipation of ion gradients. First, phosphate uptake was shown to be strongly affected by the nature of Na+ co-anions, the highest rates of uptake being observed with 100 mM-NaSCN (1.010 +/- 0.086 pmol/5 s per micrograms of protein) and the lowest with 50 mM-Na2SO4 (0.331 +/- 0.046 pmol/5 s per micrograms of protein). Anion substitution studies showed that potency of the effect of the co-anions was in the order thiocyanate greater than nitrate greater than chloride greater than isethionate greater than gluconate greater than sulphate, which correlates with the known permeability of the membrane to these anions and thus to the generation of transmembrane electrical potentials of decreasing magnitude (inside negative). The stimulation by ion-diffusion-induced potential was observed from pH 6.5 to 8.5, indicating that the transport of both monovalent and divalent phosphate was affected. In addition, inside-negative membrane potentials were generated by valinomycin-induced diffusion of K+ from K+-loaded vesicles and showed a 57% stimulation of phosphate uptake, at pH 7.5. Similar experiments with H+-loaded vesicles, in the presence of carbonyl cyanide m-chlorophenylhydrazone gave a 50% stimulation compared with controls. Inside-positive membrane potentials were also induced by reversal of the K+ gradient (outside greater than inside) in the presence of valinomycin and gave 58% inhibition of phosphate uptake. The membrane-potential dependency of phosphate uptake was finally analysed under thermodynamic equilibrium, and a stimulation by inside-negative potential was observed. The transport of phosphate was thus driven against a concentration gradient by a membrane potential, implicating the net transfer of a positive charge during the translocation process. These results indicate a major contribution of electrical potential to phosphate uptake in renal brush-border membranes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amstutz M., Mohrmann M., Gmaj P., Murer H. Effect of pH on phosphate transport in rat renal brush border membrane vesicles. Am J Physiol. 1985 May;248(5 Pt 2):F705–F710. doi: 10.1152/ajprenal.1985.248.5.F705. [DOI] [PubMed] [Google Scholar]
- Bindels R. J., van den Broek L. A., van Os C. H. Effect of pH on the kinetics of Na+-dependent phosphate transport in rat renal brush-border membranes. Biochim Biophys Acta. 1987 Feb 12;897(1):83–92. doi: 10.1016/0005-2736(87)90317-8. [DOI] [PubMed] [Google Scholar]
- Booth A. G., Kenny A. J. A rapid method for the preparation of microvilli from rabbit kidney. Biochem J. 1974 Sep;142(3):575–581. doi: 10.1042/bj1420575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunette M. G., Beliveau R., Chan M. Effect of temperature and pH on phosphate transport through brush border membrane vesicles in rats. Can J Physiol Pharmacol. 1984 Feb;62(2):229–234. doi: 10.1139/y84-034. [DOI] [PubMed] [Google Scholar]
- Burckhardt G., Stern H., Murer H. The influence of pH on phosphate transport into rat renal brush border membrane vesicles. Pflugers Arch. 1981 May;390(2):191–197. doi: 10.1007/BF00590206. [DOI] [PubMed] [Google Scholar]
- Burg M., Green N. Bicarbonate transport by isolated perfused rabbit proximal convoluted tubules. Am J Physiol. 1977 Oct;233(4):F307–F314. doi: 10.1152/ajprenal.1977.233.4.F307. [DOI] [PubMed] [Google Scholar]
- Burnham C., Munzesheimer C., Rabon E., Sachs G. Ion pathways in renal brush border membranes. Biochim Biophys Acta. 1982 Mar 8;685(3):260–272. doi: 10.1016/0005-2736(82)90066-9. [DOI] [PubMed] [Google Scholar]
- Béliveau R., Brunette M. G. The renal brush border membrane in man. Protein pattern, inorganic phosphate binding and transport: comparison with other species. Ren Physiol. 1984;7(2):65–71. [PubMed] [Google Scholar]
- Béliveau R., Strevey J. The sodium gradient induces conformational changes in the renal phosphate carrier. J Biol Chem. 1987 Dec 15;262(35):16885–16891. [PubMed] [Google Scholar]
- Béliveau R., Strévey J. Kinetic model for phosphate transport in renal brush-border membranes. Am J Physiol. 1988 Mar;254(3 Pt 2):F329–F336. doi: 10.1152/ajprenal.1988.254.3.F329. [DOI] [PubMed] [Google Scholar]
- Cheng L., Sacktor B. Sodium gradient-dependent phosphate transport in renal brush border membrane vesicles. J Biol Chem. 1981 Feb 25;256(4):1556–1564. [PubMed] [Google Scholar]
- Danisi G., Murer H., Straub R. W. Effect of pH on phosphate transport into intestinal brush-border membrane vesicles. Am J Physiol. 1984 Feb;246(2 Pt 1):G180–G186. doi: 10.1152/ajpgi.1984.246.2.G180. [DOI] [PubMed] [Google Scholar]
- Dennis V. W., Brazy P. C. Sodium, phosphate, glucose, bicarbonate, and alanine interactions in the isolated proximal convoluted tubule of the rabbit kidney. J Clin Invest. 1978 Aug;62(2):387–397. doi: 10.1172/JCI109140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis V. W., Stead W. W., Myers J. L. Renal handling of phosphate and calcium. Annu Rev Physiol. 1979;41:257–271. doi: 10.1146/annurev.ph.41.030179.001353. [DOI] [PubMed] [Google Scholar]
- Dousa T. P., Kempson S. A. Regulation of renal brush border membrane transport of phosphate. Miner Electrolyte Metab. 1982 Mar;7(3):113–121. [PubMed] [Google Scholar]
- Hammerman M. R., Schwab S. J. Phosphate transport in the kidney, studies with isolated membrane vesicles. Prog Clin Biol Res. 1984;168:325–330. [PubMed] [Google Scholar]
- Hoffmann N., Thees M., Kinne R. Phosphate transport by isolated renal brush border vesicles. Pflugers Arch. 1976 Mar 30;362(2):147–156. doi: 10.1007/BF00583641. [DOI] [PubMed] [Google Scholar]
- Hopfer U., Nelson K., Perrotto J., Isselbacher K. J. Glucose transport in isolated brush border membrane from rat small intestine. J Biol Chem. 1973 Jan 10;248(1):25–32. [PubMed] [Google Scholar]
- Kessler M., Semenza G. The small-intestinal Na+, D-glucose cotransporter: an asymmetric gated channel (or pore) responsive to delta psi. J Membr Biol. 1983;76(1):27–56. doi: 10.1007/BF01871452. [DOI] [PubMed] [Google Scholar]
- Murer H., Burckhardt G. Membrane transport of anions across epithelia of mammalian small intestine and kidney proximal tubule. Rev Physiol Biochem Pharmacol. 1983;96:1–51. doi: 10.1007/BFb0031006. [DOI] [PubMed] [Google Scholar]
- Sabolić I., Burckhardt G. Effect of the preparation method on Na+-H+ exchange and ion permeabilities in rat renal brush-border membranes. Biochim Biophys Acta. 1984 May 16;772(2):140–148. doi: 10.1016/0005-2736(84)90037-3. [DOI] [PubMed] [Google Scholar]
- Samarzija I., Molnar V., Frömter E. pH--dependence of phosphate absorption in rat renal proximal tubule. Proc Eur Dial Transplant Assoc. 1983;19:779–783. [PubMed] [Google Scholar]
- Strevey J., Brunette M. G., Béliveau R. Effect of arginine modification on kidney brush-border-membrane transport activity. Biochem J. 1984 Nov 1;223(3):793–802. doi: 10.1042/bj2230793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright E. M. Electrophysiology of plasma membrane vesicles. Am J Physiol. 1984 Apr;246(4 Pt 2):F363–F372. doi: 10.1152/ajprenal.1984.246.4.F363. [DOI] [PubMed] [Google Scholar]


