Case Presentation
A 71-year-old man with a history of recurrent tonsillar squamous cell carcinoma was admitted to the hospital with oropharyngeal bleeding. He received high-dose radiation therapy with curative intent. On day 4 of hospitalization, he demonstrated hypoxia resulting from an airway mucus plug and was brought to the medical ICU.
His past medical history was significant for resection of bilateral tonsils and soft palate followed by chemotherapy and radiation therapy. This was complicated by vocal cord paralysis, diaphragmatic dysfunction, and chronic aspiration, for which he previously had undergone placement of a percutaneous endoscopic gastrostomy tube and tracheostomy.
While in the medical ICU, wide swings in BP were noted. Systolic BP ranged from 60 to 70 mm Hg at its trough to 200 to 230 mm Hg at its peak within hours or sometimes even minutes. He remained asymptomatic during the hypertensive episodes, but became lightheaded and lethargic during hypotensive episodes. He also demonstrated a dramatic response to several antihypertensives, including metoprolol, labetalol, captopril, and hydralazine, during periods of hypertension. He likewise demonstrated a pronounced response to fluid boluses, norepinephrine, and phenylephrine during periods of hypotension. Even gentle interventions for either hypotension or hypertension often led to overcorrection to the other hemodynamic extreme.
Physical Examination Findings
BP varied from 74 to 223 mm Hg systolic to 43 to 87 mm Hg diastolic. Heart rate (HR) ranged from 67 to 100 beats/min, but most of the time was 70 to 80 beats/min, respiratory rate was 18 breaths/min, and oxyhemoglobin saturation was 95% while with 40% Fio2.
Orthostatic testing revealed a supine BP of 155 mm Hg systolic and 52 mm Hg diastolic and HR of 83 beats/min, followed by a sitting BP of 74 mm Hg systolic and 43 mm Hg diastolic and HR of 81 beats/min. Standing vital signs were not measured because of the patient’s frailty and clinical condition.
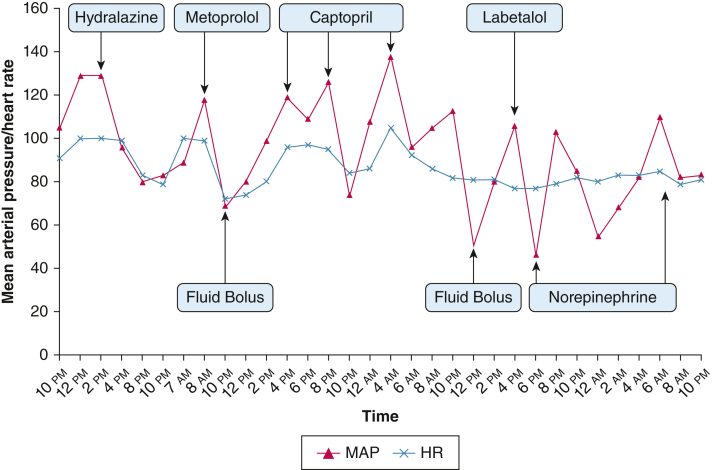
Fluid boluses or vasopressor administration resulted in rapid lability in BP (Fig 1). For example, on initial administration of phenylephrine, BP increased from 98 mm Hg systolic and 41 mm Hg diastolic to 176 mm Hg systolic and 65 mm Hg diastolic, although HR only changed from 81 to 92 beats/min.
Figure 1.
Graph showing MAP and HR responses after several hemodynamic interventions. HR = heart rate; MAP = mean arterial pressure.
During episodes of hypertension, he was not in distress and not diaphoretic. Physical examination at these times was significant for: absent soft palate, thick oral secretions, no jugular venous distention, clear breath sounds, normal HR with regular rhythm, no murmur or extra heart sounds, soft abdomen without any palpable masses, and warm and well-perfused extremities. Neurologic examination results were negative for gross deficits.
During episodes of hypotension, examination findings were notable only for fatigue and a normal HR without murmurs or extra heart sounds. His extremities remained warm. He was not cyanotic, nor did he show any focal neurologic deficits.
Diagnostic Studies
Initial laboratory results as follows: hemoglobin was 7.7 g/dL (reference, 11.5-15.5 g/dL), WBC count was 5.1 k/μL (reference, 3.7-11 k/μL), and platelet count was 259 k/μL (reference, 150-400 k/μL). Sodium was 135 mM (reference, 136-144 mM), potassium was 4.1 mM (reference, 3.7-5.1 mM), bicarbonate was 37 mM (reference, 22-30 mM), BUN was 16 mg/dL (reference, 7-21 mg/dL), and creatinine was 0.67 mg/dL (reference, 0.58-0.96 mg/dL). Alanine transaminase was 54 U/L (reference, 7-38 U/L), aspartate aminotransferase was 28 U/L (reference, 13-35 U/L), and total bilirubin was 0.2 mg/dL (reference, 0.2-1.3 mg/dL). Arterial pH was 7.46 (reference, 7.35-7.45), partial pressure of carbon dioxide was 58 mm Hg (reference, 36-46 mm Hg), and partial pressure of oxygen was 149 mm Hg (reference, 85-95 mm Hg) at 40% Fio2.
Electrocardiography showed normal sinus rhythm, normal axis, and no chamber hypertrophy. Echocardiography showed a normal left ventricle size and ejection fraction of 65% with mid cavitary obliteration. No wall motion abnormalities were noted. The right ventricle and inferior vena cava were not seen. Mild mitral, tricuspid, and aortic regurgitation were noted. The aorta was of normal size. No pericardial effusion was present.
PET imaging showed negative results for adrenal masses. Renal vascular ultrasound showed negative results for renal artery stenosis. CT imaging of the brain did not show any brain stem lesions.
What is the diagnosis?
Diagnosis: Mixed afferent and efferent baroreceptor failure secondary to carotid sinus damage resulting from radiation therapy
Discussion
Baroreflex failure is an uncommon and likely underdiagnosed cause of labile BP. It is identified primarily in patients with a history of neck surgery, trauma, or radiation that can result in damage to the baroreflex arc. The baroreceptor reflex is responsible for maintaining BP within the physiologic range on a minute-to-minute basis. Key stretch receptors are located within the carotid sinus and aortic arch and within the heart itself that transmit information on BP to the brainstem through the glossopharyngeal and vagus nerves. This is the afferent limb of the reflex. If an increase in BP is detected in the baroreceptors and relayed to the brainstem, the central parasympathetic output to the effector organs is increased, whereas the sympathetic output is decreased. This is the efferent limb. The overall effect is peripheral vasodilation, a decrease in HR and stroke volume, and, consequently, a decrease in BP. The opposite response occurs for hypotension, triggered by the same receptors and transmitted by the same neural pathway.
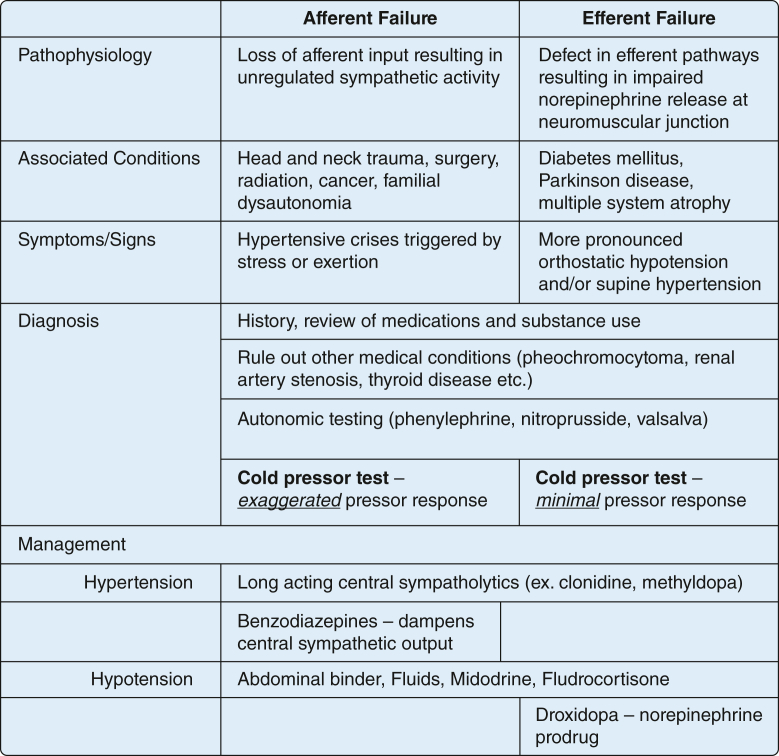
Damage to this system results in loss of this feedback mechanism and leads to extreme BP swings. The predominant clinical presentation depends on the affected limbs of the reflex and the degree of damage. In afferent limb dysfunction, the classic presentation is labile hypertension associated with tachycardia, diaphoresis, flushing, and other signs of sympathetic activation, which can resemble pheochromocytoma clinically (Fig 2). Orthostatic hypotension also is seen, but usually to a lesser degree than in other types of autonomic dysfunction. Efferent limb dysfunction, in contrast, presents with more pronounced orthostatic hypotension, decreased HR variability, and a less pronounced response to certain reflex tests, such as the cold pressor test (Fig 2).
Figure 2.
Diagram comparing afferent vs efferent baroreflex failure.
Diagnosis of this disorder relies on an antecedent history of an inciting cause and ruling out other causes such as pheochromocytoma, medication side effects, and renal artery stenosis (Fig 2). Autonomic testing then identifies a defect in the reflex arc and confirms the diagnosis. The full array of autonomic tests can be extensive and can pose some technical difficulty for patients in the ICU. However, several bedside maneuvers can be performed in the ICU such as the cold pressor test, Valsalva maneuver, and vasopressor response with phenylephrine and nitroprusside. The lack of reciprocal change in HR with these interventions that affect peripheral vascular resistance confirms baroreflex failure.
Treatment primarily is directed toward preventing the extremes of hypertension or hypotension (Fig 2). With afferent reflex failure, hypertension is mediated primarily by increased central sympathetic output. Consequently, long-acting sympatholytics such as methyldopa and transdermal clonidine can be used. Benzodiazepines can decrease cortical sympathetic stimulation. Vasodilators and short-acting oral clonidine should be avoided because of profound hypotension and rebound hypertension, respectively. In patients with hypotensive episodes, increasing intravascular volume with fluids or fludrocortisone or increasing vascular tone with midodrine is recommended (Fig 2). In patients with efferent limb dysfunction, agents such as droxidopa, an oral prodrug of norepinephrine, have been shown to be efficacious in addressing hypotensive episodes (Fig 2). In afferent limb dysfunction, sympathomimetic medications usually are avoided because they may precipitate hypertensive crises.
Clinical Course
The patient’s ICU course was characterized by predominant episodes of hypotension without a reflex HR response. After ruling out pheochromocytoma, the constellation of findings pointed to the diagnosis of baroreflex failure, namely, the dramatic response to hemodynamic interventions and lack of reflex HR decrease with phenylephrine in the context of the history of neck radiation. Mixed afferent and efferent dysfunction, with predominantly efferent reflex arc dysfunction, was suggested by the absence of tachycardia during hypotensive episodes, minimal HR variability, and the predominance of hypotension toward the latter part of the hospital course. Therefore, he received a trial of droxidopa to treat the hypotension. Shortly after, the hypotension improved. Throughout the hospitalization, he remained intermittently hypertensive, but to milder degree requiring no specific intervention.
Clinical Pearls
-
1.
Baroreflex failure is an underrecognized cause of labile BP and should be considered in patients with an antecedent history of neck trauma, surgery, or radiation.
-
2.
Diagnosis relies on history and autonomic reflex testing. Certain maneuvers such as Valsalva, cold pressor test, or phenylephrine or nitroprusside boluses can be performed while the patient is hospitalized. Absence of a reciprocal HR response to these tests confirm baroreflex failure.
-
3.
The goal of treatment is to attenuate the variability in BP. In afferent reflex failure, centrally acting sympatholytics are the first choice for controlling hypertension, whereas agents that increase vascular tone or intravascular volume such as midodrine or fludrocortisone can be used for hypotension.
Financial/Nonfinancial Disclosures
None declared.
Suggested Readings
- Robertson D., Hollister A.S., Biaggioni I., Netterville J.L., Mosqueda-Garcia R., Robertson R.M. The diagnosis and treatment of baroreflex failure. N Engl J Med. 1993;329(20):1449–1455. doi: 10.1056/NEJM199311113292003. [DOI] [PubMed] [Google Scholar]
- Ketch T., Biaggioni I., Robertson R., Robertson D. Four faces of baroreflex failure. Circulation. 2002;105(21):2518–2523. doi: 10.1161/01.cir.0000017186.52382.f4. [DOI] [PubMed] [Google Scholar]
- Heusser K., Tank J., Luft F.C., Jordan J. Baroreflex failure. Hypertension. 2005;45(5):834–839. doi: 10.1161/01.HYP.0000160355.93303.72. [DOI] [PubMed] [Google Scholar]
- Zar T., Peixoto A.J. Paroxysmal hypertension due to baroreflex failure. Kidney Int. 2008;74(1):126–131. doi: 10.1038/ki.2008.30. [DOI] [PubMed] [Google Scholar]
- Kaufmann H., Freeman R., Biaggioni I., et al. Droxidopa for neurogenic orthostatic hypotension: a randomized, placebo-controlled, phase 3 trial. Neurology. 2014;83(4):328–335. doi: 10.1212/WNL.0000000000000615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biaggioni I., Shibao C.A., Diedrich A., Muldowney J.A.S., Laffer C.L., Jordan J. Blood pressure management in afferent baroreflex failure: JACC review topic of the week. J Am Coll Cardiol. 2019;74(23):2939–2947. doi: 10.1016/j.jacc.2019.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann H., Norcliffe-Kaufmann L., Palma J.A. Baroreflex dysfunction. N Engl J Med. 2020;382(2):163–178. doi: 10.1056/NEJMra1509723. [DOI] [PubMed] [Google Scholar]
- Norcliffe-Kaufmann L., Millar Vernetti P., Palma J.A., Balgobin B.J., Kaufmann H. Afferent baroreflex dysfunction: decreased or excessive signaling results in distinct phenotypes. Semin Neurol. 2020;40(05):540–549. doi: 10.1055/s-0040-1713892. [DOI] [PubMed] [Google Scholar]
- Norcliffe-Kaufmann L., Palma J.A. Blood pressure instability in head and neck cancer survivors. Clin Auton Res. 2020;30(4):291–293. doi: 10.1007/s10286-020-00711-3. [DOI] [PMC free article] [PubMed] [Google Scholar]