Abstract
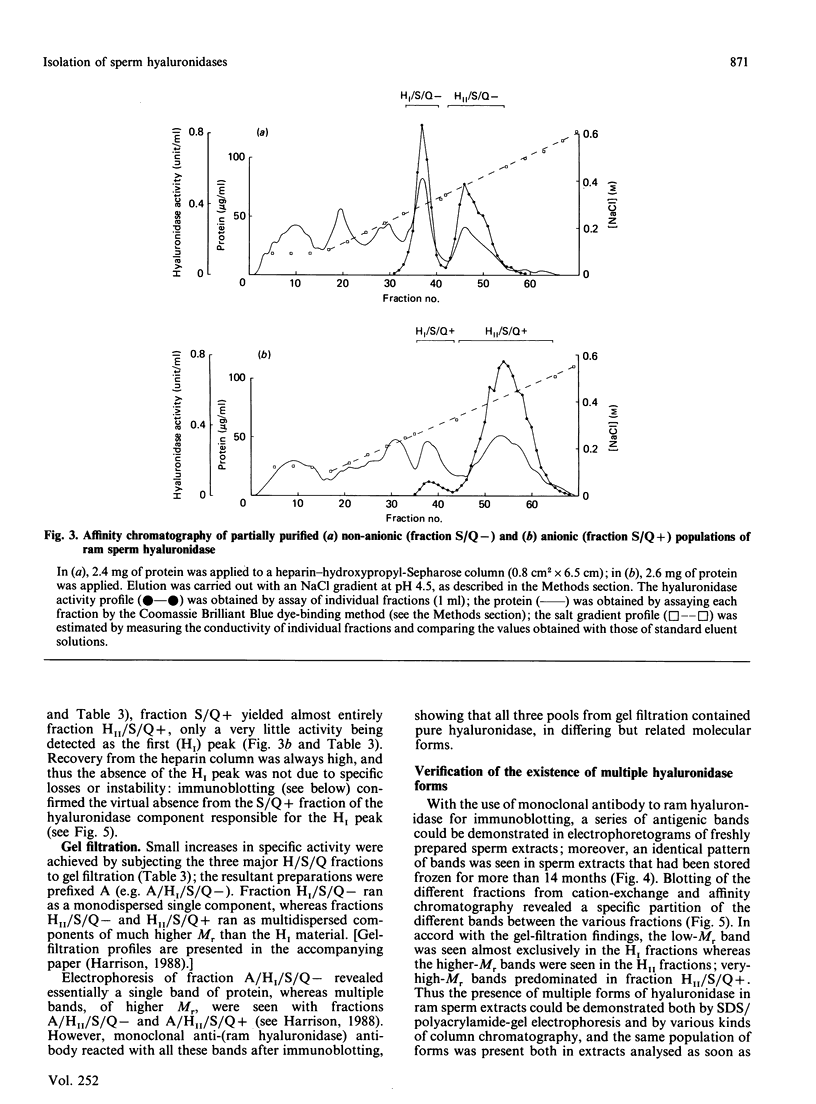
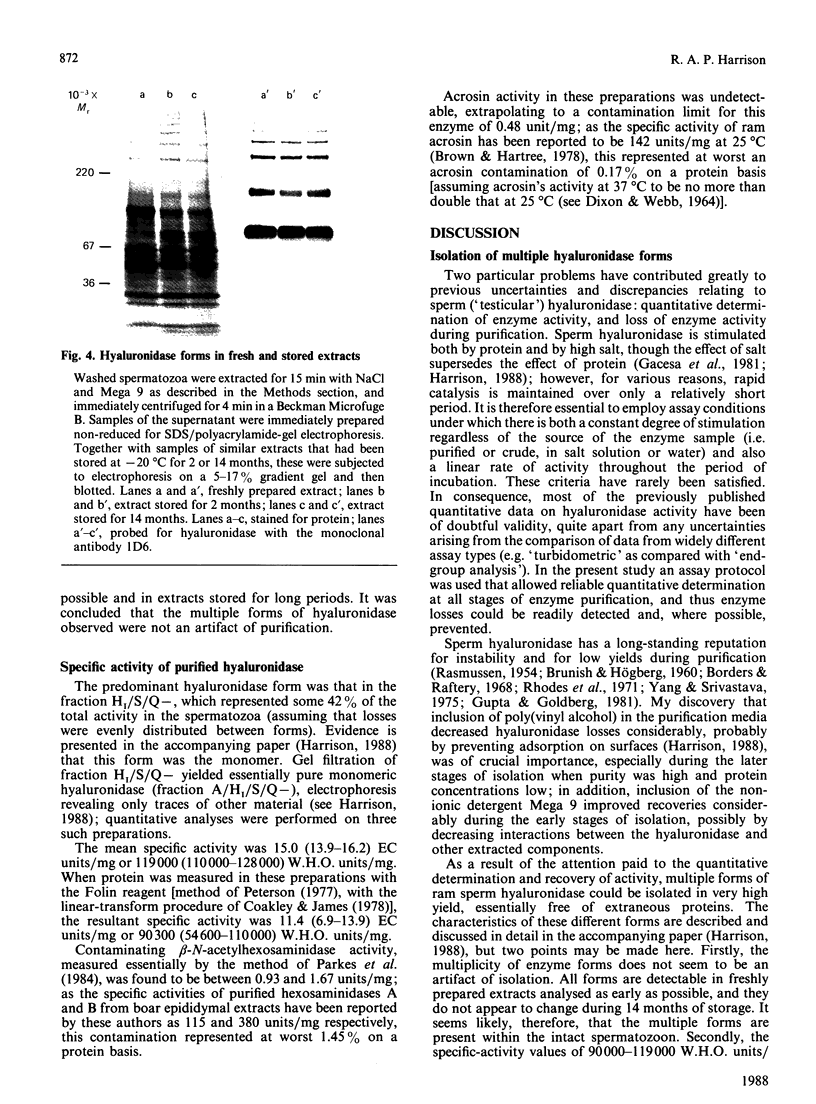
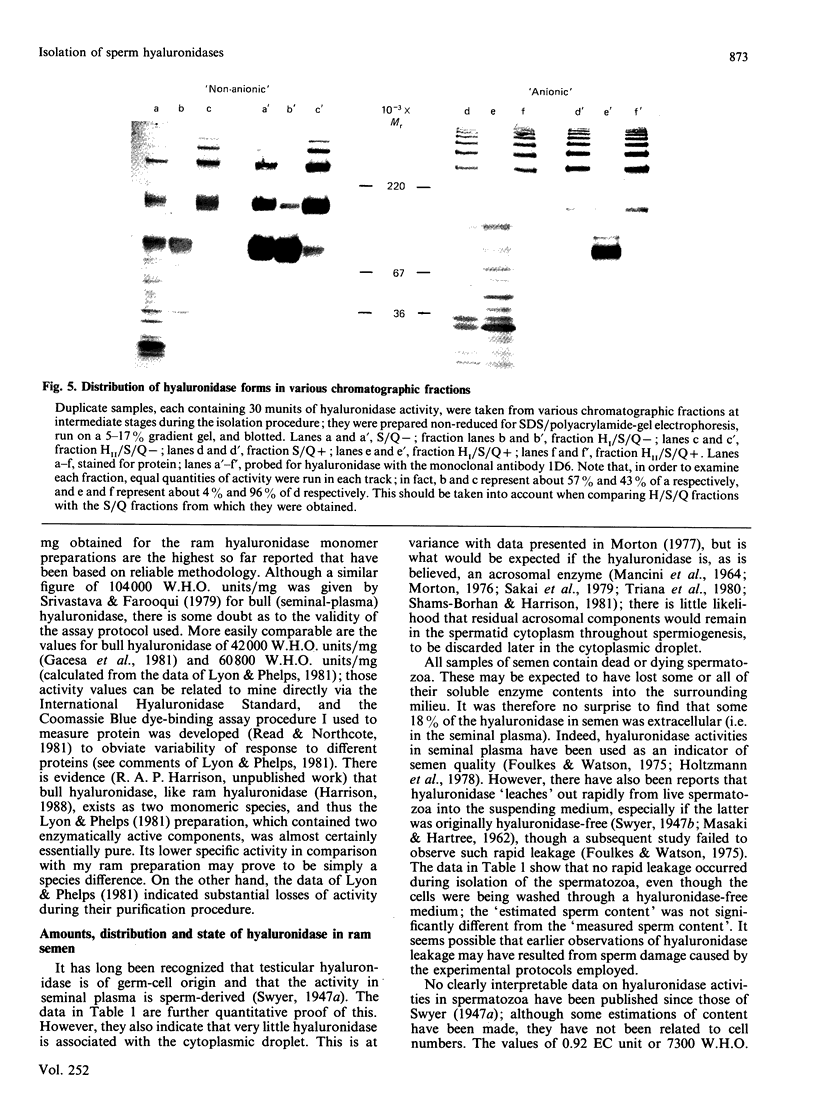
A study was made of hyaluronidase in ram semen. The end-group assay conditions used to determine activity quantitatively were chosen to ensure reliability as well as sensitivity [Gacesa, Savitsky, Dodgson & Olavesen (1981) Anal. Biochem. 118, 76-84]; they led to 1 W.H.O. Standard International Hyaluronidase Unit displaying 0.1263 EC munit (1 EC unit of activity releases 1 mumol equivalent of N-acetylglucosamine end groups/min at 37 degrees C). All the activity in the semen was shown to be sperm-derived, and intact spermatozoa were estimated to contain 1.23 EC units per 10(9) cells. In a low-ionic-strength medium, only some 20% of the hyaluronidase was extractable, although up to 80% of the activity could be extracted as the ionic strength was increased; further addition of detergent extracted the remainder. During purification of the enzyme, it was found that inclusion of poly(vinyl alcohol) in the media stabilized the activity; detergent inclusion also improved the yield, especially during early stages. As a consequence both of reliable quantitative determination and of stabilization, a number of forms of hyaluronidase could be isolated in high yield, by using anion-exchange chromatography, cation-exchange chromatography, affinity chromatography and gel filtration. The existence of all these forms was confirmed by electrophoresis and immunoblotting with the use of a monoclonal anti-(ram hyaluronidase) antibody, and their presence in very freshly prepared sperm extracts was demonstrated. The specific activity of the isolated major hyaluronidase form was 15.0 EC units/mg; this was equivalent to 119,000 W.H.O. units/mg, higher than any other previously reported values.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bansal M. R., Kanwar K. C., Gupta G. S. Immunoenzymic studies on testicular hyaluronidase from rhesus monkeys (Macaca mulatta). J Reprod Fertil. 1982 Mar;64(2):267–273. doi: 10.1530/jrf.0.0640267. [DOI] [PubMed] [Google Scholar]
- Borders C. L., Jr, Raftery M. A. Purification and partial characterization of testicular hyaluronidase. J Biol Chem. 1968 Jul 10;243(13):3756–3762. [PubMed] [Google Scholar]
- Brown C. R. Distribution of hyaluronidase in the ram spermatozoon. J Reprod Fertil. 1975 Dec;45(3):537–539. doi: 10.1530/jrf.0.0450537. [DOI] [PubMed] [Google Scholar]
- Brown C. R., Harrison R. A. The activation of proacrosin in spermatozoa from ram bull and boar. Biochim Biophys Acta. 1978 Sep 11;526(1):202–217. doi: 10.1016/0005-2744(78)90305-4. [DOI] [PubMed] [Google Scholar]
- Brown C. R., Hartree E. F. Studies on ram acrosin. Activation of proacrosin accompanying the isolation of acrosin from spermatozoa, and purification of the enzyme by affinity chromatography. Biochem J. 1978 Oct 1;175(1):227–238. doi: 10.1042/bj1750227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Coakley W. T., James C. J. A simple linear transform for the Folin-Lowry protein calibration curve to 1.0 mg/ml. Anal Biochem. 1978 Mar;85(1):90–97. doi: 10.1016/0003-2697(78)90278-6. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- Foulkes J. A., Watson P. A. Hyaluronidase activity in seminal plasma as a method of assessing bull sperm integrity. J Reprod Fertil. 1975 May;43(2):349–353. doi: 10.1530/jrf.0.0430349. [DOI] [PubMed] [Google Scholar]
- Gacesa P., Savitsky M. J., Dodgson K. S., Olavesen A. H. A recommended procedure for the estimation of bovine testicular hyaluronidase in the presence of human serum. Anal Biochem. 1981 Nov 15;118(1):76–84. doi: 10.1016/0003-2697(81)90159-7. [DOI] [PubMed] [Google Scholar]
- Gould S. F., Bernstein M. H. The localisation of bovine sperm hyaluronidase. Differentiation. 1975 Aug 11;3(1-3):123–132. doi: 10.1111/j.1432-0436.1975.tb00852.x. [DOI] [PubMed] [Google Scholar]
- Gupta G. S., Goldberg E. Isolation, properties, immunological specificity and localization of mouse testicular hyaluronidase. Biochim Biophys Acta. 1981 Feb 13;657(2):364–373. doi: 10.1016/0005-2744(81)90322-3. [DOI] [PubMed] [Google Scholar]
- HUMPHREY J. H. International standard for hyaluronidase. Bull World Health Organ. 1957;16(2):291–294. [PMC free article] [PubMed] [Google Scholar]
- Harrison R. A., Dott H. M., Foster G. C. Bovine serum albumin, sperm motility, and the "dilution effect'. J Exp Zool. 1982 Jul 20;222(1):81–88. doi: 10.1002/jez.1402220111. [DOI] [PubMed] [Google Scholar]
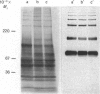
- Harrison R. A., Gaunt S. J. Multiple forms of ram and bull sperm hyaluronidase revealed by using monoclonal antibodies. J Reprod Fertil. 1988 Mar;82(2):777–785. doi: 10.1530/jrf.0.0820777. [DOI] [PubMed] [Google Scholar]
- Harrison R. A. Preliminary characterization of the multiple forms of ram sperm hyaluronidase. Biochem J. 1988 Jun 15;252(3):875–882. doi: 10.1042/bj2520875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes R., Niday E., Gordon J. A dot-immunobinding assay for monoclonal and other antibodies. Anal Biochem. 1982 Jan 1;119(1):142–147. doi: 10.1016/0003-2697(82)90677-7. [DOI] [PubMed] [Google Scholar]
- Hildreth J. E. N-D-Gluco-N-methylalkanamide compounds, a new class of non-ionic detergents for membrane biochemistry. Biochem J. 1982 Nov 1;207(2):363–366. doi: 10.1042/bj2070363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn J., Wilchek M. A new approach (cyano-transfer) for cyanogen bromide activation of Sepharose at neutral pH, which yields activated resins, free of interfering nitrogen derivatives. Biochem Biophys Res Commun. 1982 Aug;107(3):878–884. doi: 10.1016/0006-291x(82)90604-0. [DOI] [PubMed] [Google Scholar]
- Kohn J., Wilchek M. Procedures for the analysis of cyanogen bromide-activated Sepharose or Sephadex by quantitative determination of cyanate esters and imidocarbonates. Anal Biochem. 1981 Aug;115(2):375–382. doi: 10.1016/0003-2697(81)90020-8. [DOI] [PubMed] [Google Scholar]
- Kowit J. D., Maloney J. Protein cleavage by boiling in sodium dodecyl sulfate prior to electrophoresis. Anal Biochem. 1982 Jun;123(1):86–93. doi: 10.1016/0003-2697(82)90627-3. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lyon M., Phelps C. F. A rapid purification of bovine testicular hyaluronidase by chromatography on dermatan sulphate-substituted 1,6-diaminohexane--sepharose 4B. Biochem J. 1981 Nov 1;199(2):419–426. doi: 10.1042/bj1990419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANCINI R. E., ALONSO A., BARQUET J., ALVAREZ B., NEMIROVSKY M. HISTO-IMMUNOLOGICAL LOCALIZATION OF HYALURONIDASE IN THE BULL TESTIS. J Reprod Fertil. 1964 Dec;8:325–330. doi: 10.1530/jrf.0.0080325. [DOI] [PubMed] [Google Scholar]
- MASAKI J., HARTREE E. F. Distribution of metabolic activity, phospholipid and hyaluronidase between the heads and tails of bull spermatozoa. Biochem J. 1962 Aug;84:347–353. doi: 10.1042/bj0840347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton D. B. Lysosomal enzymes in mammalian spermatozoa. Front Biol. 1976;45:203–255. [PubMed] [Google Scholar]
- Nishikawa A. H., Bailon P. Affinity purification methods. Nonspecific adsorption of proteins due to ionic groups in cyanogen bromide treated agarose. Arch Biochem Biophys. 1975 Jun;168(2):576–584. doi: 10.1016/0003-9861(75)90289-1. [DOI] [PubMed] [Google Scholar]
- Nobuhara M., Sakamaki M., Takemura M., Onishi H., Suzuki Y. [The purification and the characteristics of testicular hyaluronidase (author's transl)]. Yakugaku Zasshi. 1980 Aug;100(8):832–838. doi: 10.1248/yakushi1947.100.8_832. [DOI] [PubMed] [Google Scholar]
- O'Carra P., Barry S., Griffin T. Spacer arms in affinity chromatography: use of hydrophilic arms to control or eliminate nonbiospecific adsorption effects. FEBS Lett. 1974 Jul 15;43(2):169–175. doi: 10.1016/0014-5793(74)80993-2. [DOI] [PubMed] [Google Scholar]
- Parkes H. C., Stirling J. L., Calvo P. Affinity purification and subunit structures of beta-N-acetylhexosaminidases A and B from boar epididymis. Biochem J. 1984 May 1;219(3):1009–1015. doi: 10.1042/bj2191009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Read S. M., Northcote D. H. Minimization of variation in the response to different proteins of the Coomassie blue G dye-binding assay for protein. Anal Biochem. 1981 Sep 1;116(1):53–64. doi: 10.1016/0003-2697(81)90321-3. [DOI] [PubMed] [Google Scholar]
- Rhodes C., Dodgson K. S., Olavesen A. H., Högberg B. Unsuitability of indoxyl acetate as a substrate for the assay of testicular hyaluronidase. Biochem J. 1971 May;122(4):575–582. doi: 10.1042/bj1220575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava P. N., Farooqui A. A. Heparin-sepharose affinity chromatography for purification of bull seminal-plasma hyaluronidase. Biochem J. 1979 Dec 1;183(3):531–537. doi: 10.1042/bj1830531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stambaugh R., Buckley J. Comparative studies of the acrosomal enzymes of rabbit, rhesus monkey, and human spermatozoa. Biol Reprod. 1970 Dec;3(3):275–282. doi: 10.1093/biolreprod/3.3.275. [DOI] [PubMed] [Google Scholar]
- Swyer G. I. The hyaluronidase content of semen. Biochem J. 1947;41(3):409–413. doi: 10.1042/bj0410409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swyer G. I. The release of hyaluronidase from spermatozoa. Biochem J. 1947;41(3):413–417. doi: 10.1042/bj0410413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot P., DiCarlantonio G., Zao P., Penkala J., Haimo L. T. Motile cells lacking hyaluronidase can penetrate the hamster oocyte cumulus complex. Dev Biol. 1985 Apr;108(2):387–398. doi: 10.1016/0012-1606(85)90042-9. [DOI] [PubMed] [Google Scholar]
- Talbot P., Franklin L. E. Hamster sperm hyaluronidase. II. Its release from sperm in vitro in relation to the degenerative and normal acrosome reaction. J Exp Zool. 1974 Sep;189(3):321–332. doi: 10.1002/jez.1401890305. [DOI] [PubMed] [Google Scholar]
- Talbot P., Franklin L. E. The release of hyaluronidase from guinea-pig spermatozoa during the course of the normal acrosome reaction in vitro. J Reprod Fertil. 1974 Aug;39(2):429–432. doi: 10.1530/jrf.0.0390429. [DOI] [PubMed] [Google Scholar]
- Triana L. R., Babcock D. F., Lorton S. P., First N. L., Lardy H. A. Release of acrosomal hyaluronidase follows increased membrane permeability to calcium in the presumptive capacitation sequence for spermatozoa of the bovine and other mammalian species. Biol Reprod. 1980 Aug;23(1):47–59. doi: 10.1095/biolreprod23.1.47. [DOI] [PubMed] [Google Scholar]
- Yang C. H., Srivastava P. N. Purification and properties of hyaluronidase from bull sperm. J Biol Chem. 1975 Jan 10;250(1):79–83. [PubMed] [Google Scholar]
- Zaneveld L. J., Polakoski K. L., Schumacher G. F. Properties of acrosomal hyaluronidase from bull spermatozoa. Evidence for its similarity to testicular hyaluronidase. J Biol Chem. 1973 Jan 25;248(2):564–570. [PubMed] [Google Scholar]
- Zao P. Z., Meizel S., Talbot P. Release of hyaluronidase and beta-N-acetylhexosaminidase during in vitro incubation of hamster sperm. J Exp Zool. 1985 Apr;234(1):63–74. doi: 10.1002/jez.1402340109. [DOI] [PubMed] [Google Scholar]